
302962-49-8
- Product Name:Dasatinib int
- Molecular Formula:C22H26ClN7O2S
- Purity:99%
- Molecular Weight:488.013
Product Details
Reputable Factory Supply Wholesale Dasatinib int 302962-49-8 Customized Supply
- Molecular Formula:C22H26ClN7O2S
- Molecular Weight:488.013
- Appearance/Colour:pale-yellow solid
- Melting Point:275-286 °C
- PKA:10.94±0.70(Predicted)
- PSA:134.75000
- Density:1.409 g/cm3
- LogP:3.46240
Dasatinib(Cas 302962-49-8) Usage
|
Leukemia drug |
Dasatinib, developed and marketed by Bristol Myers, is the first approved oral tyrosine kinase inhibitor which binds to multiple conformations of ABL kinase for the treatment of two leukemia indications: chronic myeloid leukemia (CML) and Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL). Dasatinib is a highly potent, ATPcompetitive ATPcompetitive kinase inhibitor which, at nanomolar concentrations, inhibits BCR-ABL, SRC family, c-KIT, EPHA2 and PDGFR-B. Dasatinib is an oral potent oncogenic kinase inhibitor, which can block signal of cancer cell replication acceleration , in May 2009, the US Food and Drug Administration (FDA) formally approved the sale of dasatinib , It has been clinically used for the treatment of various chronic myeloid leukemia (CML), including treatment of chronic myeloid leukemia which is resistant or intolerant to the treating programs including imatinib mesylate., Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph + ALL) and the treatment of patients with solid tumors. October 28, 2010 ,the FDA approved dasatinib (Sprycel) of the new indication which is used for the treatment of the rare leukemia first diagnosed Philadelphia chromosome-positive called chronic myeloid leukemia (Ph + CP-CML) . The disease is a blood and bone marrow disease associated with genetic abnormality . An open-label randomized clinical trial conducted in patients with CP-CML evaluated the ?safety and efficacy of dasatinib . Common side effects include decreased activity of the bone marrow caused by red blood cells, white blood cells and platelets decreased (myelosuppression), fluid retention, diarrhea, headache, musculoskeletal pain, and rash. October 11, 2011, the US Food and Drug Administration (FDA) said the leukemia drug dasatinib (Sprycel, Bristol-Myers Squibb Company) might increase pulmonary arterial hypertension (PAH) risk. |
|
Chemical properties |
Dasatinib is a white to off-white powder and has a melting point of 280°-286° C. The drug substance is insoluble in water and slightly soluble in ethanol and methanol. SPRYCEL? (dasatinib) is an inhibitor of multiple tyrosine kinases.SPRYCEL tablets are white to offwhite, biconvex, film-coated tablets containing dasatinib, with the following inactive ingredients: lactose monohydrate, microcrystalline cellulose, croscarmellose sodium, hydroxypropyl cellulose, and magnesium stearate. The tablet coating consists of hypromellose, titanium dioxide, and polyethylene glycol.https://www.accessdata.fda.gov/drugsatfda_docs/label/2006/021986lbl.pdf |
|
Uses |
Suitable for treatment of chronic myeloid leukemia which is resistant or intolerant to the treating programs including imatinib mesylate.Dasatinib is used to treat a certain type of chronic myeloid leukemia (CML; a type of cancer of the white blood cells) as a first treatment and in people who can no longer benefit from other leukemia medications including imatinib (Gleevec) or in those who cannot take these medications because of side effects. Dasatinib is also used to treat a certain type of chronic CML in children. Dasatinib is also used to treat a certain type of acute lymphoblastic leukemia (ALL; a type of cancer of the white blood cells) in people who can no longer benefit from other leukemia medications or who cannot take these medications because of side effects. Dasatinib is in a class of medications called kinase inhibitors. It works by blocking the action of an abnormal protein that signals cancer cells to multiply. This helps stop the spread of cancer cells.https://medlineplus.gov/druginfo/meds/a607063.html |
|
Chemical Properties |
Pale-Yellow Solid |
|
Brand name |
Sprycel (Bristol-Myers Squibb). |
|
Clinical Use |
#N/A |
|
Metabolism |
Dasatinib is extensively metabolised, mainly via the cytochrome P450 isoenzyme CYP3A4, forming an active metabolite.Elimination is predominantly in the faeces, mostly as metabolites. Following a single oral dose of [14C]-labelled dasatinib, approximately 89% of the dose was eliminated within 10 days, with 4% and 85% of the radioactivity recovered in the urine and faeces, respectively. Unchanged dasatinib accounted for 0.1% and 19% of the dose in urine and faeces, respectively, with the remainder of the dose as metabolites. |
InChI:InChI=1/C22H26ClN7O2S.H2O/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31;/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27);1H2
302962-49-8 Relevant articles
Discovery of N-(2-chloro-6-methylphenyl)-2-(6-(4-(2-hydroxyethyl)- piperazin-1-yl)-2-methylpyrimidin-4-ylamino)thiazole-5-carboxamide (BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays
Lombardo, Louis J.,Lee, Francis Y.,Chen, Ping,Norris, Derek,Barrish, Joel C.,Behnia, Kamelia,Castaneda, Stephen,Cornelius, Lyndon A. M.,Das, Jagabandhu,Doweyko, Arthur M.,Fairchild, Craig,Hunt, John T.,Inigo, Ivan,Johnston, Kathy,Kamath, Amrita,Kan, David,Klei, Herbert,Marathe, Punit,Pang, Suhong,Peterson, Russell,Pitt, Sidney,Schieven, Gary L.,Schmidt, Robert J.,Tokarski, John,Wen, Mei-Li,Wityak, John,Borzilleri, Robert M.
, p. 6658 - 6661 (2004)
A series of substituted 2-(aminopyridyl)...
Scalable and impurity-free process for dasatinib: Src and BCR-Abl inhibitor
Buchappa,Sagar Vijay Kumar,Durga Prasad,Aparna
, p. 1621 - 1628 (2018)
An efficient, telescopic, impurity-free ...
Synthesis and biological evaluation of novel dasatinib analogues as potent DDR1 and DDR2 kinase inhibitors
Liu, Lu,Hussain, Muzammal,Luo, Jinfeng,Duan, Anna,Chen, Chaonan,Tu, Zhengchao,Zhang, Jiancun
, p. 420 - 427 (2017)
Novel dasatinib analogues as DDR1 and DD...
HALOGENATED-HETEROARYL AND OTHER HETEROCYCLIC KINASE INHIBITORS, AND USES THEREOF
-
, (2021/11/04)
The invention relates to kinase inhibito...
HETEROCYCLIC KINASE INHIBITORS AND USES THEREOF
-
, (2020/05/30)
The invention relates to kinase inhibito...
302962-49-8 Upstream products
-
103-76-4
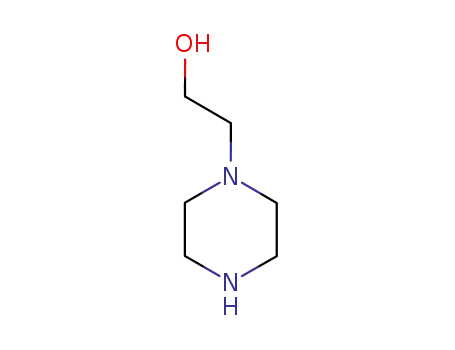
1-(2-hydroxyethyl)piperazine
-
302964-08-5
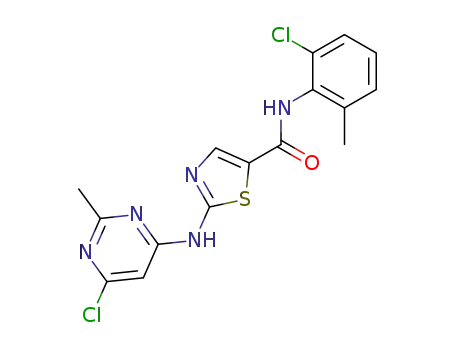
2-(6-chloro-2-methylpyrimidin-4-ylamino)-N-(2-chloro-6-methylphenyl)thiazole-5-carboxamide
-
40398-01-4
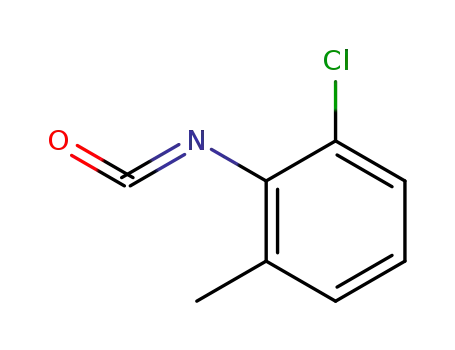
2-chloro-6-methylphenyl isocyanate
-
302964-11-0
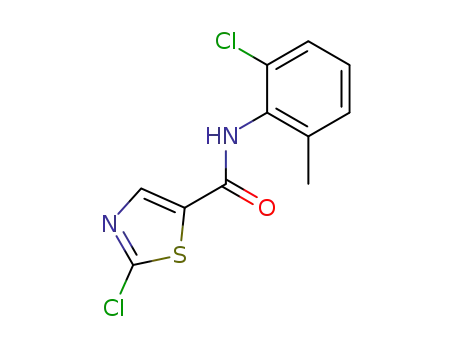
2-chloro-N-(2-chloro-6-methylphenyl)-1,3-thiazole-5-carboxamide
302962-49-8 Downstream products
-
863127-78-0
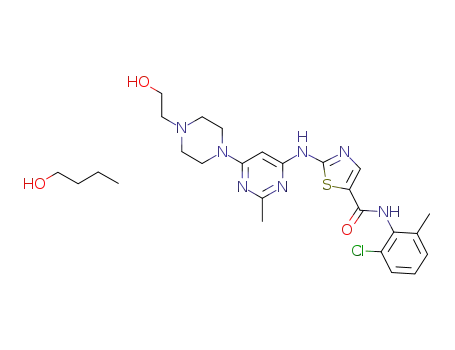
N-(2-chloro-6-methylphenyl)-2-((6-(4-(2-hydroxyethyl)-1-piperazinyl)-2-methyl-4-pyrimidinyl)amino)-1,3-thiazole-5-carboxamide butanolate
-
863127-77-9
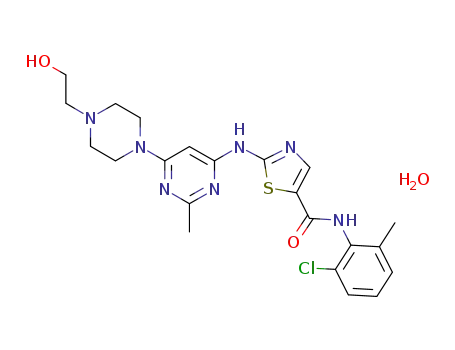
dasatinib monohydrate
-
881381-50-6
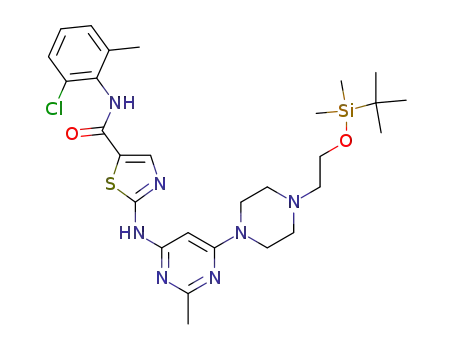
2-(6-(4-(2-(tert-butyldimethylsilyloxy)ethyl)piperazin-1-yl)-2-methylpyrimidin-4-ylamino)-N-(2-chloro-6-methylphenyl)thiazole-5-carboxamide
-
910297-52-8
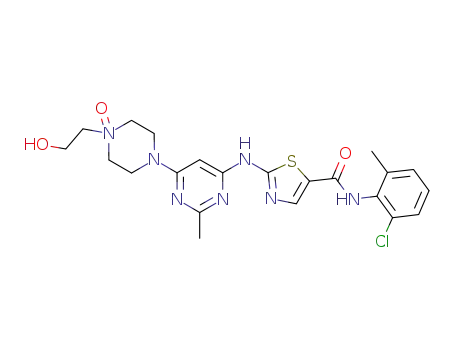
dasatinib N-oxide
Relevant Products
-
dasatinib monohydrate
CAS:863127-77-9
-
3-Amino-4-methylbenzoic acid ethyl ester
CAS:41191-92-8







