6494-19-5
- Product Name:3-Methyl-6-nitroindazole
- Molecular Formula:C8H7N3O2
- Purity:99%
- Molecular Weight:177.162
Product Details
Chinese Factory Supply Reliable Quality 3-Methyl-6-nitroindazole 6494-19-5 with Fast Shipping
- Molecular Formula:C8H7N3O2
- Molecular Weight:177.162
- Vapor Pressure:0.00684mmHg at 25°C
- Melting Point:187-188 °C
- Refractive Index:1.704
- Boiling Point:225.226 °C at 760 mmHg
- PKA:11.47±0.40(Predicted)
- Flash Point:90.014 °C
- PSA:74.50000
- Density:1.438 g/cm3
- LogP:2.30270
3-Methyl-6-nitroindazole(Cas 6494-19-5) Usage
|
Chemical Properties |
Brown solid |
|
Uses |
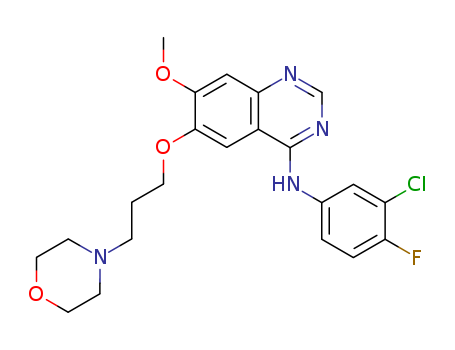
3-Methyl-6-nitroindazole a reactant used in the preparation of Pazopanib (P210925), an oral angiogenesis inhibitor targeting VEGFR and PDGFR. |
InChI:InChI=1/C8H7N3O2/c1-5-7-3-2-6(11(12)13)4-8(7)10-9-5/h2-5H,1H3
6494-19-5 Relevant articles
Preparation method of pazopanib intermediate
-
, (2021/03/24)
The invention provides a preparation met...
Method for synthesizing 3-methyl-6-nitro-1H-indazole by microchannel diazo reaction
-
Paragraph 0027; 0028; 0029; 0030; 0031-0034, (2019/03/10)
The invention discloses a method for syn...
Method for preparing 2, 3-dimethyl-2H-indazole-6-benzylamine hydrochloride
-
Paragraph 0029; 0032; 0035-0037; 0040-0042; 0045-0047, (2020/03/14)
The invention discloses a method for pre...
2,3-dimethyl-6-urea -2H-indazoles and its preparation method and application
-
Paragraph 0125-0126; 0130-0132, (2016/10/09)
The invention discloses a 2, 3-dimethyl-...
6494-19-5 Process route
-
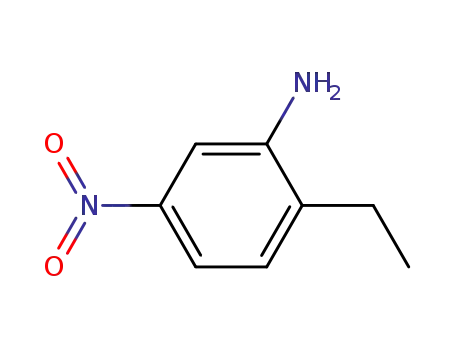
- 20191-74-6
2-ethyl-5-nitroaniline

-
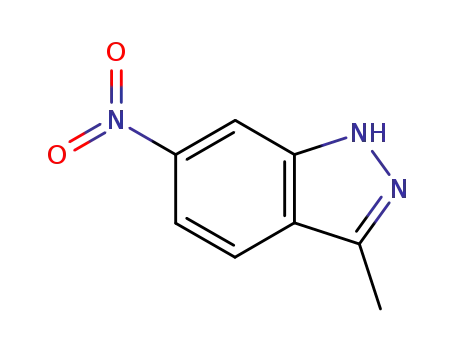
- 6494-19-5
3-methyl-6-nitro-1H-indazole
| Conditions | Yield |
|---|---|
|
With acetic acid; isopentyl nitrite; at 20 ℃; for 0.75h;
|
98% |
|
With tert.-butylnitrite; In acetic acid; at 20 ℃; for 0.75h; Inert atmosphere;
|
98% |
|
With tert.-butylnitrite; acetic acid; for 0.5h;
|
98% |
|
With acetic acid; sodium nitrite; In water; at 35 ℃; for 0.5h;
|
93.9% |
|
With acetic acid; sodium nitrite; at 0 - 25 ℃;
|
40.5% |
|
With tert.-butylnitrite; acetic acid; at 20 ℃; for 1h;
|
|
|
2-ethyl-5-nitroaniline; With tert.-butylnitrite; acetic acid; for 0.75h;
With sodium hydrogencarbonate;
|
|
|
With acetic acid; isopentyl nitrite; at 20 ℃; for 1.5h; Inert atmosphere;
|
|
|
With acetic acid; sodium nitrite; In water; at 40 ℃; for 0.025h; Temperature;
|
-
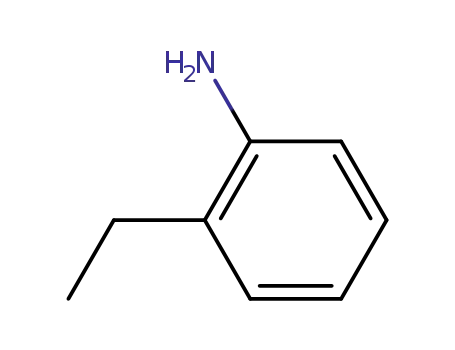
- 578-54-1
ortho-ethylaniline

-

- 6494-19-5
3-methyl-6-nitro-1H-indazole
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1: sulfuric acid; potassium nitrate / 1.5 h / 0 °C
2: acetic acid; isopentyl nitrite / 1.5 h / 20 °C / Inert atmosphere
With sulfuric acid; acetic acid; potassium nitrate; isopentyl nitrite;
|
|
|
Multi-step reaction with 2 steps
1: sulfuric acid; nitric acid / 0.5 h / 0 - 5 °C
2: tert.-butylnitrite; acetic acid / 0.5 h
With tert.-butylnitrite; sulfuric acid; nitric acid; acetic acid;
|
6494-19-5 Upstream products
-
20191-74-6

2-ethyl-5-nitroaniline
-
578-54-1

ortho-ethylaniline
-
612-22-6
2-nitro(ethylbenzene)
6494-19-5 Downstream products
-
79173-62-9
3-methyl-1H-indazol-6-amine
-
62235-34-1
3-methyl-1-(5-methyl-3-phenyl-isoxazole-4-carbonyl)-6-nitro-1H-indazole
-
62235-33-0
3-methyl-1-(3-methyl-5-phenyl-isoxazole-4-carbonyl)-6-nitro-1H-indazole
-
62235-35-2
1-[3-(2-chloro-phenyl)-5-methyl-isoxazole-4-carbonyl]-3-methyl-6-nitro-1H-indazole
Relevant Products
-
Nilotinib int
CAS:641571-10-0
-
3,4-Difluoro-2-(2-fluoro-4-iodophenylaMino)benzoic Acid
CAS:391211-97-5
-
Gefitinib int
CAS:184475-35-2