
23491-48-7
- Product Name:5-(4-Methylpiperazin-1-yl)-2-nitroaniline
- Molecular Formula:C11H16N4O2
- Purity:99%
- Molecular Weight:236.274
Product Details
Reputable Factory Supply 99% Pure 5-(4-Methylpiperazin-1-yl)-2-nitroaniline 23491-48-7 with Efficient Transportation
- Molecular Formula:C11H16N4O2
- Molecular Weight:236.274
- Vapor Pressure:4.37E-08mmHg at 25°C
- Melting Point:152-155 °C
- Boiling Point:444.2 °C at 760 mmHg
- PKA:7.35±0.42(Predicted)
- Flash Point:222.4 °C
- PSA:78.32000
- Density:1.264 g/cm3
- LogP:2.03610
5-(4-Methylpiperazin-1-yl)-2-nitroaniline(Cas 23491-48-7) Usage
|
Chemical Properties |
Solid |
InChI:InChI=1/C11H16N4O2/c1-13-4-6-14(7-5-13)9-2-3-11(15(16)17)10(12)8-9/h2-3,8H,4-7,12H2,1H3/p+1
23491-48-7 Relevant articles
A new strategy for site-specific alkylation of DNA using oligonucleotides containing an abasic site and alkylating probes
Sato, Norihiro,Tsuji, Genichiro,Sasaki, Yoshihiro,Usami, Akira,Moki, Takuma,Onizuka, Kazumitsu,Yamada, Ken,Nagatsugi, Fumi
, p. 14885 - 14888 (2015)
Selective chemical reactions with DNA, s...
Benzimidazole compound, preparation method thereof and application of the benzimidazole compound in preparation of ferroptosis inhibitor
-
Paragraph 0066; 0070-0074, (2021/06/13)
The invention discloses a benzimidazole ...
Di-(benzimidazole)-1, 2, 3-triazole derivative as well as preparation and application thereof in inflammatory dermatosis
-
Paragraph 0058-0060; 0063-0065, (2021/06/23)
The invention belongs to the technical f...
Discovery of 5-(4-methylpiperazin-1-yl)-2-nitroaniline derivatives as a new class of SIRT6 inhibitors
Chen, Xiuli,Huang, Shenzhen,Li, Linli,Li, Wenpei,Sun, Weining,Tian, Chenyu,Yang, Shengyong
supporting information, (2020/06/08)
SIRT6 is a deacetylase of histone H3 and...
Pyrazole spleen tyrosine kinase inhibitor as well as preparation method and application thereof
-
Paragraph 0256-0259; 0272-0275, (2020/12/29)
The invention discloses a pyrazole splee...
23491-48-7 Process route
-
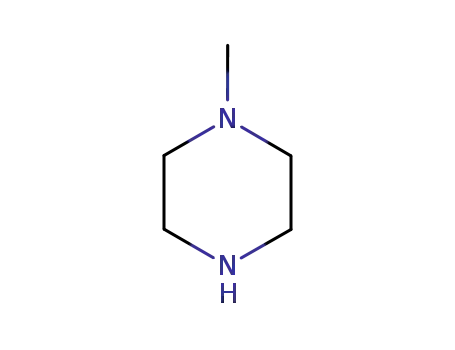
- 109-01-3
1-methyl-piperazine

-
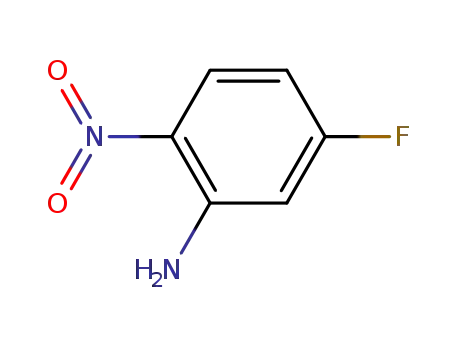
- 2369-11-1
5-fluoro-2-nitroaniline

-
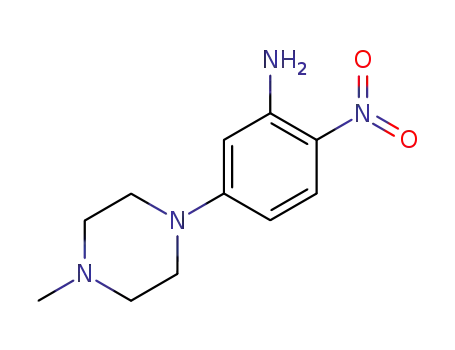
- 23491-48-7
5-(4-methylpiperazine-1-yl)-2-nitroaniline
| Conditions | Yield |
|---|---|
|
With triethylamine; In 1-methyl-pyrrolidin-2-one; at 100 ℃; for 16h;
|
96% |
|
With N-ethyl-N,N-diisopropylamine; In 1-methyl-pyrrolidin-2-one; at 120 ℃; for 4h;
|
83% |
|
With triethylamine; In 1-methyl-pyrrolidin-2-one; at 90 ℃; for 1h;
|
|
|
With triethylamine; In 1-methyl-pyrrolidin-2-one; at 100 ℃; for 3h;
|
|
|
In 1,4-dioxane; for 18h; Reflux;
|
|
|
at 60 ℃;
|
|
|
With triethylamine; In 1-methyl-pyrrolidin-2-one; at 100 ℃; for 20h; Microwave irradiation;
|
1.45 g |
|
With triethylamine; In 1-methyl-pyrrolidin-2-one; water; at 100 ℃; for 20h; Microwave irradiation;
|
1.45 g |
|
With potassium carbonate; In N,N-dimethyl-formamide; at 90 ℃; for 3h;
|
3 g |
-

- 109-01-3
1-methyl-piperazine

-
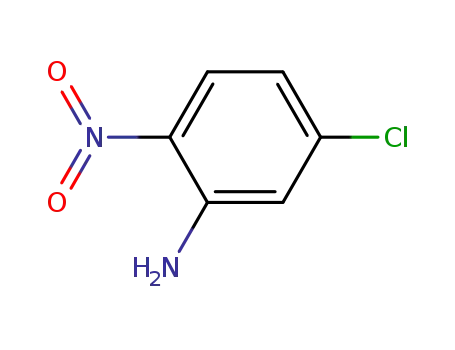
- 1635-61-6
5-chloro-2-nitroaniline

-

- 23491-48-7
5-(4-methylpiperazine-1-yl)-2-nitroaniline
| Conditions | Yield |
|---|---|
|
With potassium carbonate; In DMF (N,N-dimethyl-formamide); at 120 ℃; for 20h;
|
100% |
|
In ethanol; at 97 ℃; for 40h;
|
99% |
|
In ethanol; water; at 20 - 97 ℃; for 46h; Product distribution / selectivity;
|
99% |
|
In ethanol; at 97 ℃; for 40h; Product distribution / selectivity;
|
98.6% |
|
In ethanol; at 97 ℃; for 40h; Product distribution / selectivity;
|
98.6% |
|
With potassium carbonate; In N,N-dimethyl-formamide; at 130 ℃; Inert atmosphere;
|
98.4% |
|
In ethanol; at 20 - 97 ℃; for 42 - 43h; Product distribution / selectivity;
|
98.6% |
|
In ethanol; at 20 - 97 ℃; for 36 - 40h; Product distribution / selectivity;
|
98.6% |
|
In ethanol; at 97 ℃; for 40h; Product distribution / selectivity;
|
98.6% |
|
at 100 ℃; Product distribution / selectivity;
|
97.8% |
|
at 100 ℃; Product distribution / selectivity;
|
97.8% |
|
In water; at 110 ℃; for 7h; Product distribution / selectivity;
|
97.6% |
|
at 100 ℃; Neat (no solvent);
|
97.8% |
|
In ethanol; at 20 - 100 ℃; Product distribution / selectivity;
|
97.8% |
|
In water; at 20 - 100 ℃;
|
97.8% |
|
at 100 ℃; Product distribution / selectivity; Neat (no solvent);
|
97.8% |
|
at 100 ℃; Product distribution / selectivity;
|
97.8% |
|
With sodium chloride; In water; at 110 - 112 ℃; for 5 - 8h; Product distribution / selectivity;
|
93.9% |
|
With sodium chloride; In ethylene glycol; at 122 ℃; for 4 - 5h; Product distribution / selectivity;
|
92.7% |
|
In ethanol; at 97 ℃; for 36 - 41h; Product distribution / selectivity;
|
92.9% |
|
for 24h; Inert atmosphere; Reflux;
|
92% |
|
With sodium hydroxide; sodium chloride; In water; at 105 - 112 ℃; for 7 - 22h; Product distribution / selectivity;
|
91.1% |
|
at 130 - 140 ℃; for 2h;
|
90% |
|
With potassium carbonate; In N,N-dimethyl-formamide; at 110 ℃; for 12h; Inert atmosphere;
|
90% |
|
With potassium carbonate; In N,N-dimethyl-formamide; at 120 ℃; for 24h;
|
89% |
|
With potassium carbonate; In N,N-dimethyl-formamide; at 110 ℃; for 24h;
|
89% |
|
With potassium carbonate; In N,N-dimethyl-formamide; at 110 ℃;
|
85% |
|
With potassium carbonate; In N,N-dimethyl-formamide; at 90 ℃; for 3h;
|
82% |
|
With potassium carbonate; In dimethyl sulfoxide; at 150 ℃;
|
80% |
|
With potassium carbonate; In N,N-dimethyl-formamide; at 100 ℃; for 6h;
|
80% |
|
With potassium carbonate; In N,N-dimethyl-formamide; for 3h; Heating;
|
79% |
|
With potassium carbonate; In N,N-dimethyl-formamide; at 100 ℃; for 6h;
|
79% |
|
With potassium carbonate; In N,N-dimethyl-formamide; at 120 ℃; for 18h;
|
78% |
|
With potassium carbonate; In N,N-dimethyl-formamide; at 110 ℃; for 36h;
|
78% |
|
With potassium carbonate; In N,N-dimethyl-formamide; for 24h; Heating;
|
75% |
|
With potassium carbonate; In N,N-dimethyl-formamide; for 16h; Inert atmosphere;
|
72.8% |
|
With potassium carbonate; In N,N-dimethyl-formamide; at 110 ℃; for 16h;
|
71% |
|
With potassium carbonate; In N,N-dimethyl-formamide; at 120 ℃; for 18.5h;
|
67% |
|
In N,N-dimethyl-formamide; at 100 ℃; for 5h;
|
60% |
|
With potassium carbonate; In N,N-dimethyl-formamide; at 110 ℃; for 12h; Inert atmosphere;
|
60% |
|
5-chloro-2-nitroaniline; With triethylamine; In 1-methyl-pyrrolidin-2-one; at 20 ℃; for 0.5h;
1-methyl-piperazine; In 1-methyl-pyrrolidin-2-one; at 160 ℃; for 16h;
|
56% |
|
With potassium carbonate; In N,N-dimethyl-formamide; at 110 ℃; for 5h;
|
55% |
|
With potassium carbonate; In N,N-dimethyl-formamide; at 100 ℃; for 24h;
|
27% |
|
With potassium carbonate; In N,N-dimethyl-formamide; at 90 ℃;
|
|
|
In N2; water;
|
670 g (97.8%) |
|
at 130 ℃; for 24 - 48h;
|
|
|
In N,N-dimethyl-formamide; at 120 ℃; for 24h;
|
|
|
With potassium carbonate; In N,N-dimethyl-formamide; at 110 ℃;
|
|
|
With potassium carbonate; at 50 ℃; for 48h; Inert atmosphere;
|
|
|
With potassium carbonate; In N,N-dimethyl-formamide; at 160 ℃; for 21h;
|
23491-48-7 Upstream products
-
109-01-3

1-methyl-piperazine
-
1635-61-6

5-chloro-2-nitroaniline
-
68-12-2
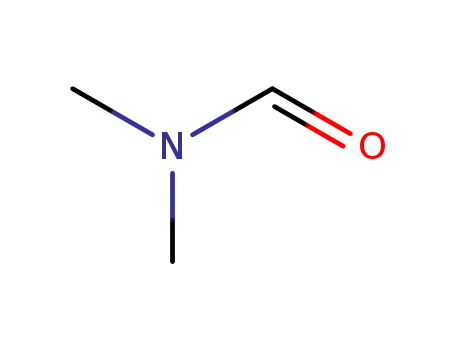
N,N-dimethyl-formamide
-
2369-11-1

5-fluoro-2-nitroaniline
23491-48-7 Downstream products
-
81215-93-2
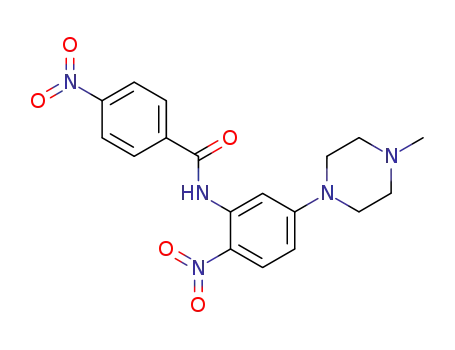
N-<5-(4-Methyl-1-piperazinyl)-2-nitrophenyl>-4-nitro-benzamide
-
54998-08-2
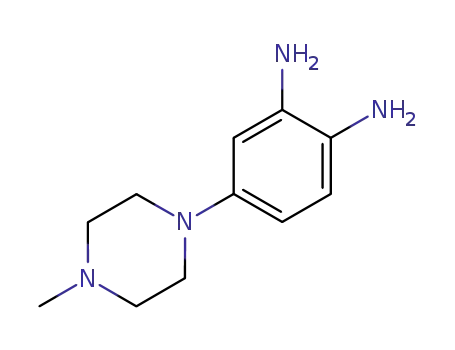
4-(4-methylpiperazino)-1,2-diaminobenzene
-
897446-64-9
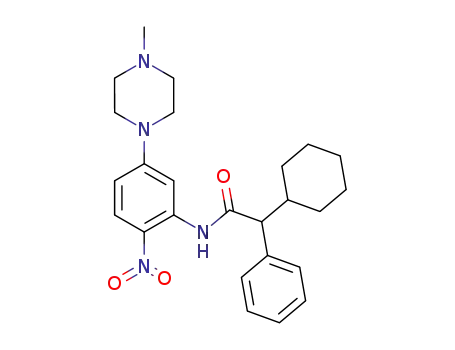
2-cyclohexyl-N-[5-(4-methylpiperazin-1-yl)-2-nitrophenyl]-2-phenylacetamide
-
402948-37-2
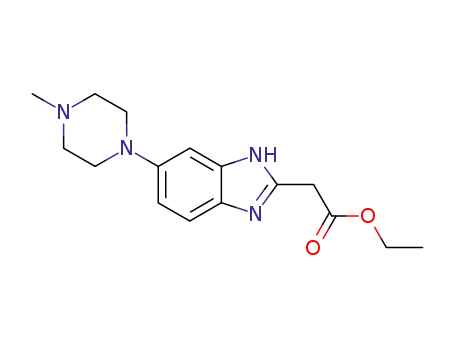
[6-(4-methylpiperazin-1-yl)-1H-benzimidazol-2-yl]acetic acid ethyl ester
Relevant Products
-
Nilotinib int
CAS:641571-10-0
-
Methyl 3-amino-4-methylbenzoate
CAS:18595-18-1
-
3,4-Difluoro-2-(2-fluoro-4-iodophenylaMino)benzoic Acid
CAS:391211-97-5




