641569-96-2
- Product Name:3-[(Aminoiminomethyl)amino]-4-methylbenzoic acid ethyl ester mononitrate
- Molecular Formula:C11H15N3O2.HNO3
- Purity:99%
- Molecular Weight:284.272
Product Details
Quality Factory Supply 99% Pure 3-[(Aminoiminomethyl)amino]-4-methylbenzoic acid ethyl ester mononitrate 641569-96-2 with Efficient Shipping
- Molecular Formula:C11H15N3O2.HNO3
- Molecular Weight:284.272
- Melting Point:197.0 to 201.0 °C
- PSA:154.25000
- LogP:2.52560
3-[(Aminoiminomethyl)amino]-4-methylbenzoic acid ethyl ester mononitrate(Cas 641569-96-2) Usage
|
General Description |
3-[(Aminoiminomethyl)amino]-4-methylbenzoic acid ethyl ester mononitrate is a chemical compound that is an ethyl ester of a mononitrate derivative of 3-[(aminoiminomethyl)amino]-4-methylbenzoic acid. It has a complex molecular structure that includes multiple functional groups, such as an amino group, a nitrate group, and a benzoic acid group. The compound is likely to be used in pharmaceutical or chemical research due to its unique structure and potential properties, possibly for its role in drug development or analysis. Further study and research are necessary to fully understand the potential applications and properties of this compound. |
InChI:InChI=1/C11H15N3O2.HNO3/c1-3-16-10(15)8-5-4-7(2)9(6-8)14-11(12)13;2-1(3)4/h4-6H,3H2,1-2H3,(H4,12,13,14);(H,2,3,4)
641569-96-2 Relevant articles
Method for preparing N-(5-carboxyl-2-methylphenyl)-4-(3-pyridine)-2-pyrilamine
-
Paragraph 0033; 0034, (2021/05/05)
The invention discloses a method for pre...
Design, synthesis and biological evaluation of pyridin-3-yl pyrimidines as potent Bcr-Abl inhibitors
Pan, Xiaoyan,Dong, Jinyun,Gao, Hongping,Wang, Fang,Zhang, Yanmin,Wang, Sicen,Zhang, Jie
, p. 592 - 599 (2014/05/06)
A series of pyridin-3-yl pyrimidines was...
N-PHENYL-2-PYRIMIDINE-AMINE DERIVATIVES AND PROCESS FOR THE PREPARATION THEREOF
-
Page/Page column 10, (2008/06/13)
The present invention relates to a novel...
INHIBITORS OF TYROSINE KINASES
-
Page 37, (2008/06/13)
The invention relates to compounds of fo...
641569-96-2 Process route
-
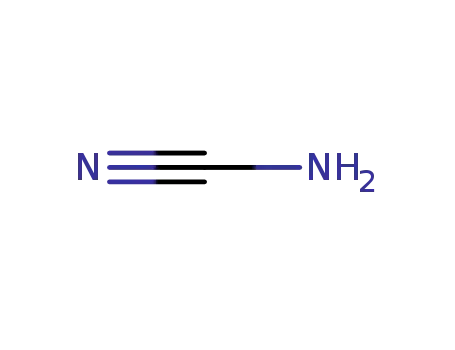
- 420-04-2
CYANAMID

-
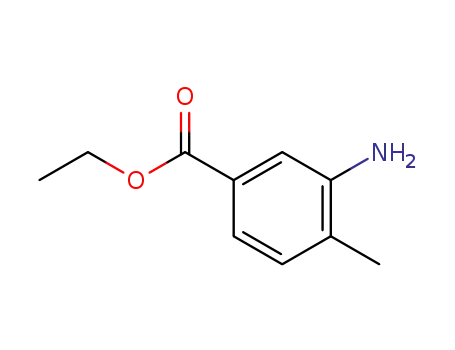
- 41191-92-8
3-amino-4-methylbenzoic acid ethyl ester

-
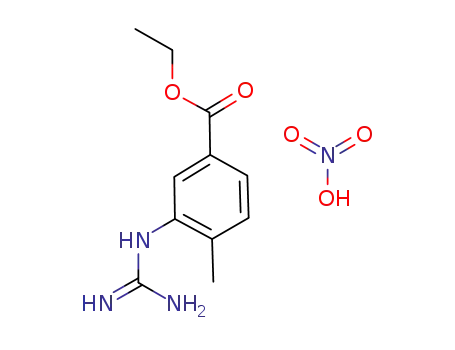
- 641569-96-2
3-guanidino-4-methylbenzoic acid ethyl ester nitrate
| Conditions | Yield |
|---|---|
|
CYANAMID; 3-amino-4-methylbenzoic acid ethyl ester; With hydrogenchloride; In ethanol; at 90 ℃; for 15.25h;
With ammonium nitrate; In water; at 5 - 10 ℃; for 1h;
|
|
|
CYANAMID; 3-amino-4-methylbenzoic acid ethyl ester; With hydrogenchloride; In ethanol; water; toluene; Reflux;
With ammonium nitrate; In ethanol; water; toluene; at 5 - 10 ℃; for 1h;
|
-
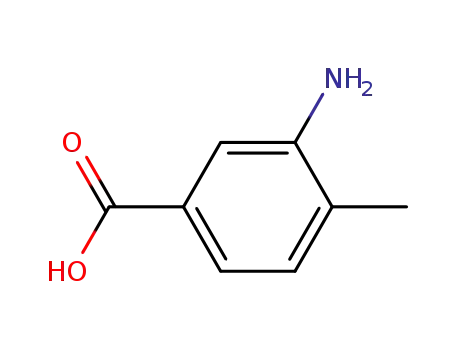
- 2458-12-0
3-amino-p-toluic acid

-

- 641569-96-2
3-guanidino-4-methylbenzoic acid ethyl ester nitrate
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 3 steps
1: sulfuric acid / 12 h / Reflux; Inert atmosphere
2: hydrogenchloride / ethanol; water / 15 h / Reflux
3: ammonium nitrate / 0.5 h / 0 °C
With ammonium nitrate; hydrogenchloride; sulfuric acid; In ethanol; water;
|
641569-96-2 Upstream products
-
420-04-2
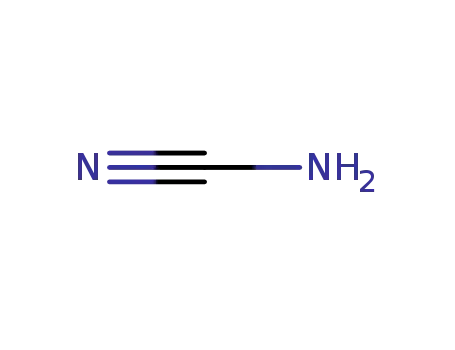
CYANAMID
-
41191-92-8

3-amino-4-methylbenzoic acid ethyl ester
-
2458-12-0

3-amino-p-toluic acid
-
641569-95-1
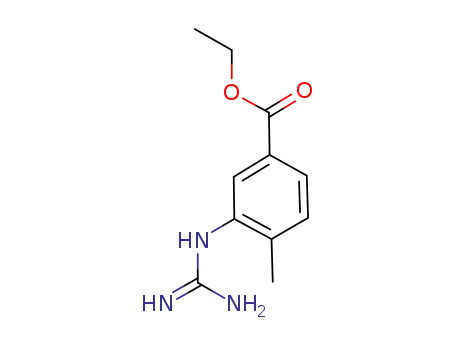
C11H15N3O2
641569-96-2 Downstream products
-
641569-97-3
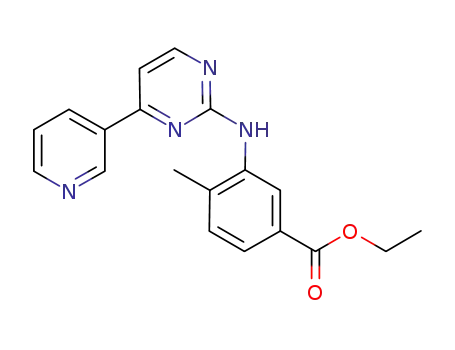
4-methyl-3-[[4-(3-pyridyl)-2-pyrimidinyl]amino]benzoic acid ethyl ester
-
1205540-16-4
N-(3-bromo-5-(trifluoromethyl)phenyl)-4-methyl-3-((4-(pyridin-3-yl)pyrimidin-2-yl)amino)benzamide
Relevant Products
-
Dasatinib int
CAS:302962-49-8
-
4-Methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]benzoic acid
CAS:641569-94-0