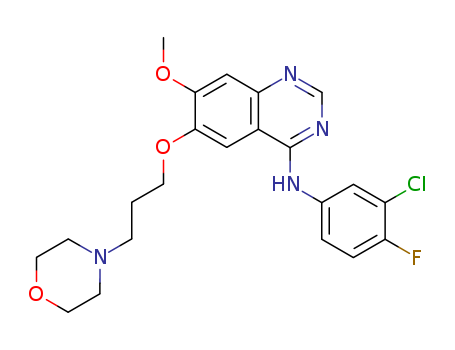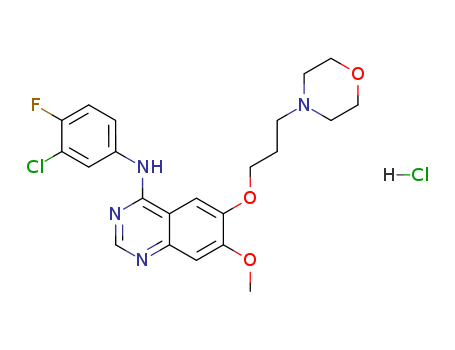
184475-55-6
- Product Name:4-(3-Chloro-4-fluorophenylamino)-7-methoxy-6-[3-(4-morpholinyl)propoxy]quinazoline hydrochloride
- Molecular Formula:C22H24ClFN4O3.HCl
- Purity:99%
- Molecular Weight:483.37
Product Details
Quality Manufacturer Supply Hot Sale 4-(3-Chloro-4-fluorophenylamino)-7-methoxy-6-[3-(4-morpholinyl)propoxy]quinazoline hydrochloride 184475-55-6 On Stock
- Molecular Formula:C22H24ClFN4O3.HCl
- Molecular Weight:483.37
- Vapor Pressure:4.9E-15mmHg at 25°C
- Boiling Point:607.7 °C at 760 mmHg
- Flash Point:321.3 °C
- PSA:68.74000
- LogP:5.08850
4-(3-Chloro-4-fluorophenylamino)-7-methoxy-6-[3-(4-morpholinyl)propoxy]quinazoline hydrochloride(Cas 184475-55-6) Usage
|
Biological Activity |
the egfr is a mr 170,000 transmembrane glycoprotein with an external binding domain and an intracellular tyrosine kinase domain. gefitinib (zd-1839, iressa) is an epidermal growth factor receptor-selective tyrosine kinase inhibitor. |
|
in vitro |
gefitinib inhibited colony formation in soft agar in a dose dependent manner in all cancer cell lines. however, treatment with higher doses resulted in a 2–4-fold increases in apoptosis. dose-dependent supra-additive increase in growth inhibition was observed when cancer cells were treated with totoxic drugs and gefitinib. the combined treatment markedly enhanced apoptotic cell death induced by single agent treatment [1]. |
|
in vivo |
gefitinib treatment of nude mice bearing established human geo colon cancer xenografts revealed a reversible dose-dependent inhibition of tumor growth because geo tumors resumed the growth rate of controls at the end of the treatment [1]. |
|
references |
[1] ciardiello f, caputo r, bianco r, damiano v, pomatico g, de placido s, bianco ar, tortora g. antitumor effect and potentiation of cytotoxic drugs activity in human cancer cells by zd-1839 (iressa), an epidermal growth factor receptor-selective tyrosine kinase inhibitor. clin cancer res. 2000;6(5):2053-63.[2] cantarini mv, mcfarquhar t, smith rp, bailey c, marshall al. relative bioavailability and safety profile of gefitinib administered as a tablet or as a dispersion preparation via drink or nasogastric tube: results of a randomized, open-label, three-period crossover study in healthy volunteers. clin ther. 2004;26(10):1630-6. |
InChI:InChI=1/C22H24ClFN4O3.ClH/c1-29-20-13-19-16(12-21(20)31-8-2-5-28-6-9-30-10-7-28)22(26-14-25-19)27-15-3-4-18(24)17(23)11-15;/h3-4,11-14H,2,5-10H2,1H3,(H,25,26,27);1H
184475-55-6 Relevant articles
Quinazoline derivatives
-
, (2008/06/13)
The invention concerns quinazoline deriv...
184475-55-6 Process route

-
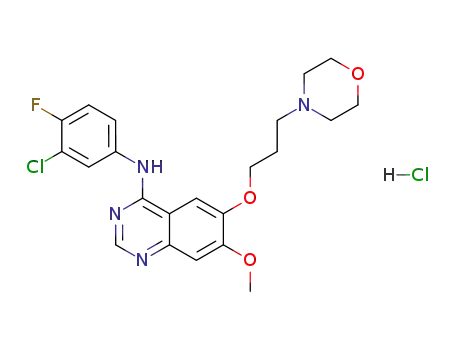
- 184475-55-6
Gefitinib hydrochloride
| Conditions | Yield |
|---|---|
|
|
-

- 184475-55-6
Gefitinib hydrochloride

-
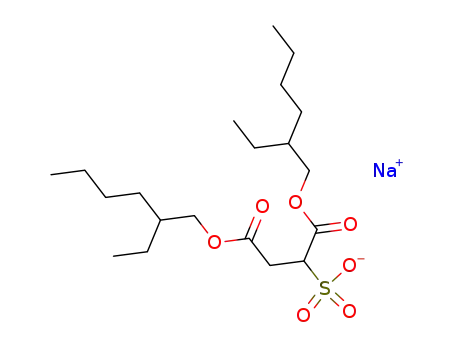
- 577-11-7
sodium docusate

-
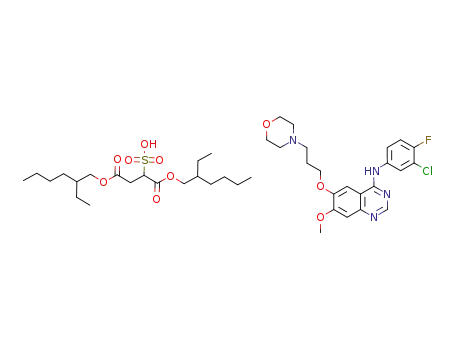
-
gefitinib docusate
| Conditions | Yield |
|---|---|
|
In dichloromethane; water; at 20 ℃;
|
96% |
Relevant Products
-
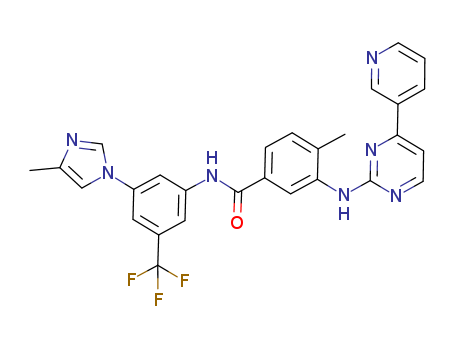
Regorafenib (Hydrochloride) int
CAS:835621-07-3
-
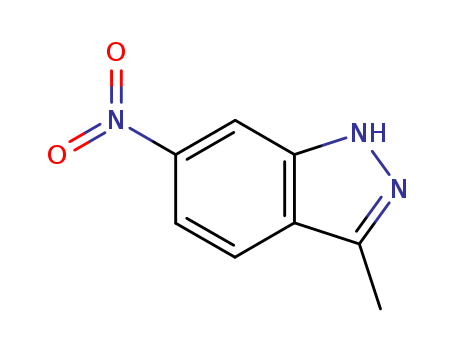
Gefitinib int
CAS:184475-35-2
-
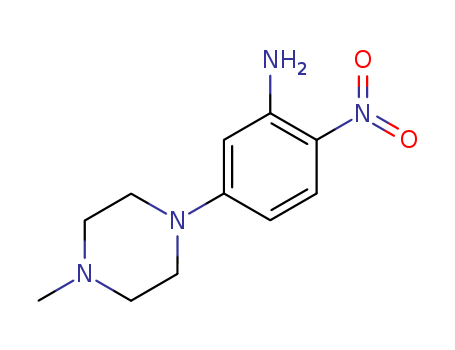
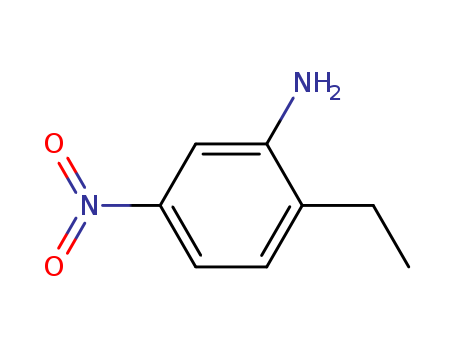
2-ethyl-5-nitroaniline
CAS:20191-74-6