
41191-92-8
- Product Name:3-Amino-4-methylbenzoic acid ethyl ester
- Molecular Formula:C10H13NO2
- Purity:99%
- Molecular Weight:179.219
Product Details
Factory Supply Top Purity 3-Amino-4-methylbenzoic acid ethyl ester 41191-92-8 On Stock
- Molecular Formula:C10H13NO2
- Molecular Weight:179.219
- Vapor Pressure:0.001mmHg at 25°C
- Melting Point:48.6-50.1℃
- Refractive Index:1.55
- Boiling Point:310.065 °C at 760 mmHg
- PKA:3.35±0.10(Predicted)
- Flash Point:163.664 °C
- PSA:52.32000
- Density:1.104 g/cm3
- LogP:2.33510
AKOS BB-3093(Cas 41191-92-8) Usage
|
General Description |
AKOS BB-3093, also known as N-(3-Aminopropyl)-Imidodicarbonimidic Diamide, is a chemical compound with the molecular formula C4H14N6. It is a diamide derivative, which is commonly used as a corrosion inhibitor in various industrial applications. AKOS BB-3093 is highly soluble in water and has a melting point of 178-180°C. AKOS BB-3093 is known for its ability to protect metal surfaces from corrosion by forming a protective film on the surface, thereby preventing the oxidation process. It is also used in the production of specialty chemicals and as a reagent in chemical synthesis processes. Overall, AKOS BB-3093 is an important chemical compound with diverse industrial applications, particularly in corrosion prevention and chemical synthesis. |
InChI:InChI=1/C10H13NO2/c1-3-13-10(12)8-5-4-7(2)9(11)6-8/h4-6H,3,11H2,1-2H3
41191-92-8 Relevant articles
Expedient Synthesis of Thioether-Functionalized Hydrotris(indazolyl)borate as an Anchoring Platform for Rotary Molecular Machines
Erbland, Guillaume,Gisbert, Yohan,Rapenne, Gwéna?l,Kammerer, Claire
, p. 4731 - 4739 (2018)
Major improvements in the synthesis of s...
Synthesis of new tripodal tri-functionalized hydrotris(indazol-1-yl)borate ligands and x-ray structures of their cyclopentadieneruthenium complexes
Carella, Alexandre,Vives, Guillaume,Cox, Tara,Jaud, Jo?l,Rapenne, Gwéna?l,Launay, Jean-Pierre
, p. 980 - 987 (2006)
Two new tripodal ligands designed to anc...
Method for preparing N-(5-carboxyl-2-methylphenyl)-4-(3-pyridine)-2-pyrilamine
-
Paragraph 0031; 0032, (2021/05/05)
The invention discloses a method for pre...
Divergent Synthesis of Molecular Winch Prototypes
Abid, Seifallah,Gisbert, Yohan,Kammerer, Claire,Rapenne, Gwéna?l
supporting information, p. 16242 - 16249 (2021/10/16)
We report the synthesis of conceptually ...
Investigations into the potential role of metabolites on the anti-leukemic activity of imatinib, nilotinib and midostaurin
Manley, Paul W.
, p. 561 - 570 (2019/09/03)
The efficacy and side-effects of drugs d...
41191-92-8 Process route
-
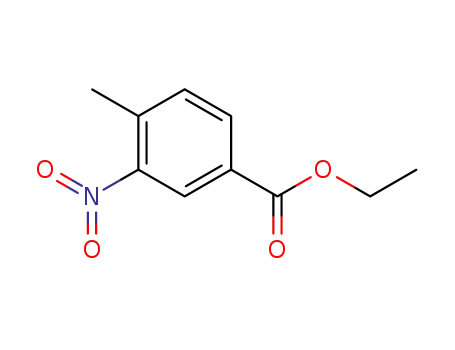
- 19013-15-1
ethyl 4-methyl-3-nitro-benzoate

-
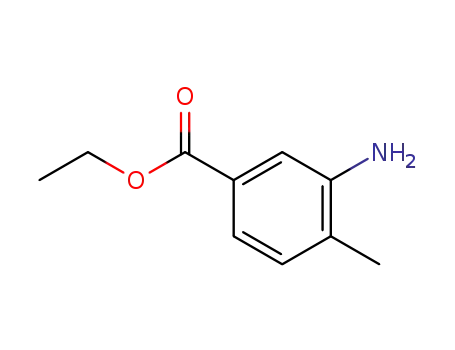
- 41191-92-8
3-amino-4-methylbenzoic acid ethyl ester
| Conditions | Yield |
|---|---|
|
With tin(ll) chloride; In ethanol; at 70 ℃; for 0.5h;
|
100% |
|
With palladium 10% on activated carbon; hydrogen; In ethanol; for 168h; under 760.051 Torr; Darkness;
|
87% |
|
With palladium 10% on activated carbon; ammonium formate; In methanol; Heating;
|
-
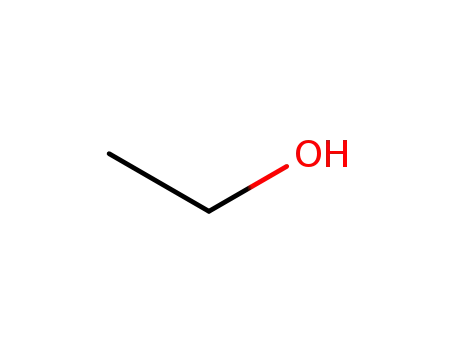
- 64-17-5
ethanol

-
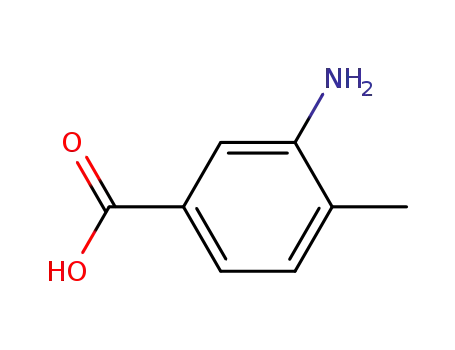
- 2458-12-0
3-amino-p-toluic acid

-

- 41191-92-8
3-amino-4-methylbenzoic acid ethyl ester
| Conditions | Yield |
|---|---|
|
With thionyl chloride; Heating;
|
98% |
|
With sulfuric acid; for 12h; Reflux; Inert atmosphere;
|
98% |
|
With thionyl chloride;
|
98% |
|
With thionyl chloride; at 90 ℃; for 16h; Schlenk technique; Inert atmosphere;
|
98% |
|
With sulfuric acid; at 75 ℃; for 31h;
|
90% |
41191-92-8 Upstream products
-
64-17-5

ethanol
-
96-98-0
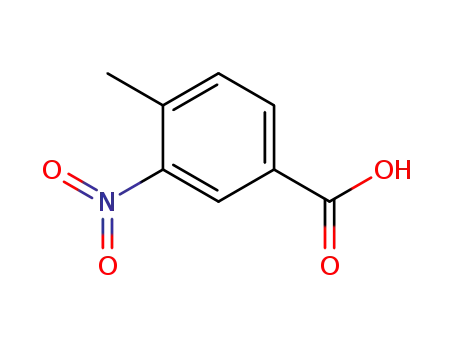
4-methyl-3-nitrobenzoic acid
-
19013-15-1

ethyl 4-methyl-3-nitro-benzoate
-
2458-12-0

3-amino-p-toluic acid
41191-92-8 Downstream products
-
99176-16-6
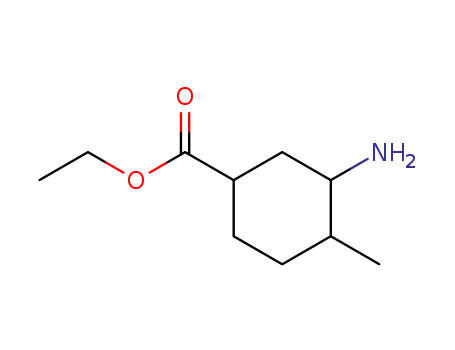
3-amino-4-methyl-cyclohexanecarboxylic acid ethyl ester
-
147962-81-0
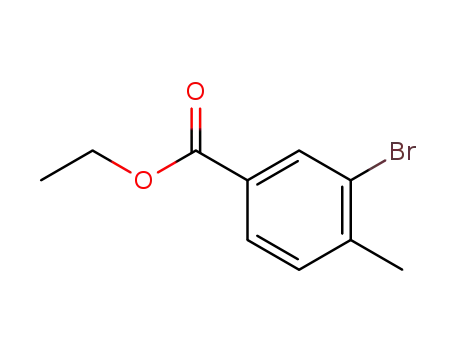
ethyl 3-bromo-4-methylbenzoate
-
713-09-7
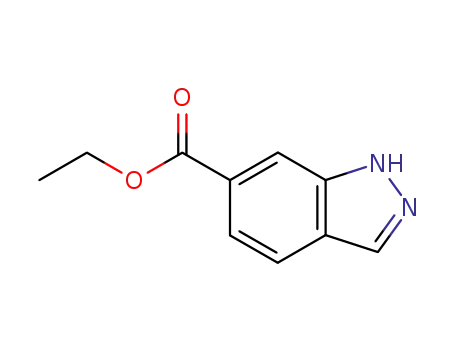
ethyl 1H-indazole-6-carboxylate
-
916902-55-1
6-(hydroxymethyl)-1H-indazole
Relevant Products
-
Nilotinib int
CAS:641571-10-0
-
Cinacalcet Hydrochloride
CAS:364782-34-3
-
dasatinib monohydrate
CAS:863127-77-9






