364782-34-3
- Product Name:Cinacalcet Hydrochloride
- Molecular Formula:C22H22F3N.HCl
- Purity:99%
- Molecular Weight:393.88
Product Details
Trustworthy Factory Supply Buy High Grade Cinacalcet Hydrochloride 364782-34-3 with the Best Price
- Molecular Formula:C22H22F3N.HCl
- Molecular Weight:393.88
- Appearance/Colour:Off-white to tan solid
- Melting Point:175-177 °C
- Boiling Point:440.9 °C at 760 mmHg
- Flash Point:220.5 °C
- PSA:12.03000
- LogP:7.33490
Cinacalcet hydrochloride(Cas 364782-34-3) Usage
|
Description |
Cinacalcet is the first entry in a new class of therapeutic agents called the calcimimetics. It was launched as an oral treatment for secondary hyperparathyroidism (SHPT) in patients with chronic kidney disease on dialysis and for hypercalcemia in patients with parathyroid carcinoma. SHPT is associated with increased parathyroid hormone (PTH) secretion, which is triggered by low serum levels of calcium resulting from the failure of the kidney to clear phosphorous from the body and its inability to produce sufficient quantities of vitamin D. The consequences of increased PTH include stimulation of osteoclastic activity, cortical bone resorption and marrow fibrosis. PTH secretion is primarily regulated by the calcium-sensing receptor (CaR), which is located on the surface of the chief cell of the parathyroid gland. Calcimimetics bind to CaR and increase the sensitivity of CaR to extracellular calcium, thereby enabling its activation at subnormal levels of serum calcium. As a result, in the presence of these agents, the low levels of endogenous calcium in patients with renal failure are able to exert a suppressive effect on PTH secretion. Parathyroid carcinoma is also associated with elevated PTH levels, which are driven by autonomous parathyroid gland activity and subsequently lead to hypercalcemia. Although surgical resection is the primary therapy for treating hypercalcemia in parathyroid carcinoma patients, calcimimetics offer a nonsurgical alternative for patients with failed parathyroidectomy, metastatic parathyroid carcinoma, or high surgical risk. The recommended dosage of cinacalcet for the treatment of SHPT in chronic kidney disease is 30mg once daily at start and subsequent titration to 60, 90, 120 or 180 mg once daily. The dosage for the treatment of hypercalcemia in patients with parathyroid carcinoma is 30 mg twice daily at start and subsequent titration to 60 or 90 mg twice daily, or 90mg three or four times daily as necessary to normalize serum calcium level. After oral administration of cinacalcet, maximum plasma concentration is achieved in approximately 2 to 6 hours. It has a terminal half-life of 30 to 40 hours and steady-state drug levels are reached within 7 days. Cinacalcet has a high volume of distribution (1000 L) and high protein binding (93%–97%). It is extensively metabolized in the liver, mainly by CYP3A4, CYP2D6 and CYP1A2. The primary routes of elimination are in the urine (80%) and in the feces (15%). In Phase III clinical trials involving 1136 patients with SHPT, administration of cinacalcet at 30–180 mg/day doses for 6 months produced 38–48% decrease in intact PTH. Overall, 64% of patients given cinacalcet achieved at least a 30% reduction in PTH, versus 11% of placebo patients. Calcium-phosphorous product was reduced 14% by the active treatment and did not change in the placebo group. In a much smaller clinical study involving 21 hypercalcemic patients with parathyroid carcinoma, administration of 60–360 mg/day doses of cinacalcet resulted in 71% of patients achieving a target reduction of ≥1 mg/dL in serum calcium. The most common adverse events in these trials were nausea and vomiting. In vitro, cinacalcet is a strong inhibitor of CYP2D6; therefore, dose adjustments may be required when coadministered with medications that are predominantly metabolized by CYP2D6 and have a narrow therapeutic index (e.g. flecainide, vinblastine, thioridazine and most tricyclic antidepressants). Cinacalcet is prepared in a two-step synthesis starting from 3-[3-(trifluoromethyl)phenyl]propionaldehyde, by first condensing with (R)-(1-naphthyl)ethylamine to form the corresponding imine and subsequent reduction of the imine with sodium cyanoborohydride. |
|
Chemical Properties |
Off-White to Tan Solid |
|
Originator |
NPS pharmaceuticals (US) |
|
Uses |
Cinacalcet hydrochloride can be used in clinical trial in secondary hyperparathyroidism. |
|
Definition |
ChEBI: A hydrochloride derived from equimolar amounts of cinacalcet and hydrogen chloride. |
|
Synthesis |
General syntheses of this class of compounds have been published, however, the specific synthesis of cinacalcet (III) has not been available to date. The synthesis of cinacalcet, based on a patented procedure, is depicted in Scheme 3. A mixture of 1-acetonaphthone (21), 3-trifluoromethyl-1-propylamine (22) and titanium (IV) isopropoxide were stirred at rt to form the enamine intermediate which was reduced with methanolic sodium cyanoborohydride at rt to give corresponding racemic a-methyl amine (23). Compound 23 was resolved and then treated with HCl etherate to give cinacalcet hydrochloride (III) as a white solid. |
|
references |
[1] ure?a p1, fraz?o jm. calcimimetic agents: review and perspectives. kidney int suppl. 2003 jun;(85):s91-6.[2] colloton m1, shatzen e, wiemann b, starnes c, scully s, henley c, martin d.cinacalcet attenuates hypercalcemia observed in mice bearing either rice h-500 leydig cell or c26-dct colon tumors. eur j pharmacol. 2013 jul 15;712(1-3):8-15. |
InChI:InChI=1/C22H22F3N.ClH/c1-16(20-13-5-10-18-9-2-3-12-21(18)20)26-14-6-8-17-7-4-11-19(15-17)22(23,24)25;/h2-5,7,9-13,15-16,26H,6,8,14H2,1H3;1H/t16-;/m1./s1
364782-34-3 Relevant articles
Preparation method of cinacalcet hydrochloride and intermediate thereof
-
Paragraph 0054-0069, (2021/05/05)
The invention discloses a preparation me...
Cinacalcet intermediate and synthesis method of cinacalcet hydrochloride
-
Paragraph 0155; 0158; 0171-0173, (2021/07/17)
The invention relates to a cinacalcet in...
Creating High Regioselectivity by Electronic Metal-Support Interaction of a Single-Atomic-Site Catalyst
Jing, Hongyu,Li, Jiong,Li, Wen-Hao,Li, Yadong,Wang, Dingsheng,Wang, Yu,Yang, Jiarui,Zhang, Jian,Zhao, Jie
, p. 15453 - 15461 (2021/09/30)
Ligands are the most commonly used means...
Preparation method of cinacalcet hydrochloride
-
, (2021/05/26)
The invention discloses a method for pre...
364782-34-3 Process route
-
![(R)-(1-(naphthalen-1-yl)-ethyl)-[3-(3-trifluoromethyl-phenyl)-prop-2-ynyl]-amine hydrochloride](/upload/2024/4/64a2794d-4a53-4302-ad63-84917d34018a.png)
- 1093944-40-1
(R)-(1-(naphthalen-1-yl)-ethyl)-[3-(3-trifluoromethyl-phenyl)-prop-2-ynyl]-amine hydrochloride

-
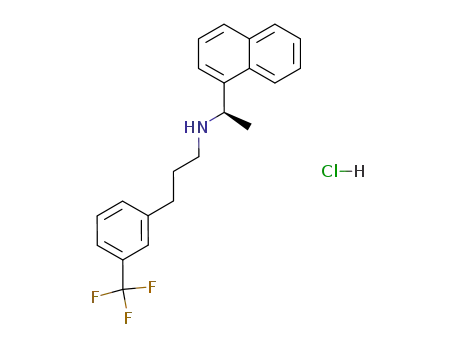
- 364782-34-3,1217809-88-5
cinacalcet hydrochloride
| Conditions | Yield |
|---|---|
|
With hydrogen; 5%-palladium/activated carbon; In water; isopropyl alcohol; at 20 ℃; for 3h;
|
90% |
|
With hydrogen; 5%-palladium/activated carbon; In isopropyl alcohol; at 20 ℃; for 3h; under 760.051 Torr;
|
90% |
-
![N-[(1R)-1-(naphthalen-1-yl)ethyl]-3-[3-(trifluoromethyl)phenyl]prop-2-en-1-amine fumarate](/upload/2024/4/418e8118-b964-4a80-83a3-40a6d44316da.png)
-
N-[(1R)-1-(naphthalen-1-yl)ethyl]-3-[3-(trifluoromethyl)phenyl]prop-2-en-1-amine fumarate

-

- 364782-34-3,1217809-88-5
cinacalcet hydrochloride
| Conditions | Yield |
|---|---|
|
With hydrogenchloride; hydrogen; 2-5percent w/w palladium on carbon; In methanol; water; at 20 - 30 ℃; Inert atmosphere;
|
83.8% |
364782-34-3 Upstream products
-
226256-56-0
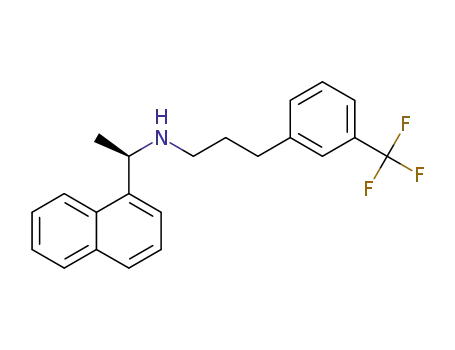
cinacalcet
-
1005450-55-4
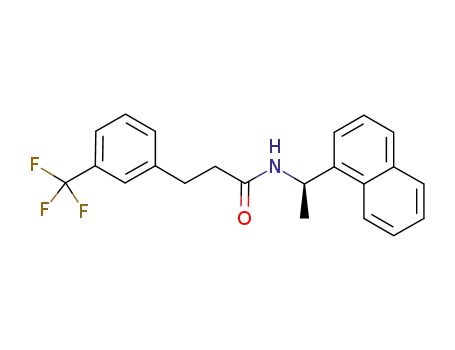
(R)‐N‐(1‐(naphthalen‐1‐yl)ethyl)‐3‐(3‐trifluoromethylphenyl)propanamide
-
1095393-66-0
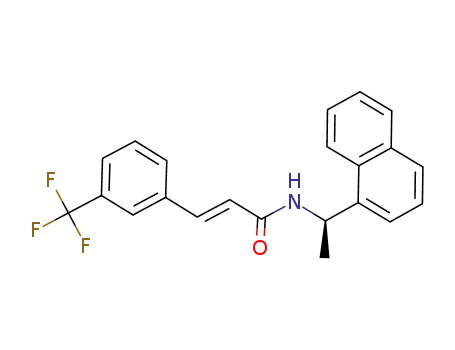
(R)-N-(1-naphthalen-1-yl-ethyl)-3-(3-trifluoromethyl-phenyl)-(E)-acrylamide
-
1025064-29-2
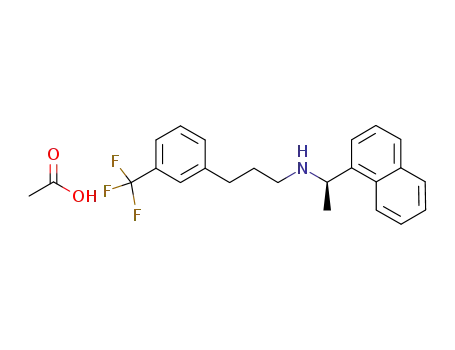
N-[1-(R)-(-)-(1-naphthyl)ethyl]-3-[3-(trifluoromethyl)phenyl]-1-aminopropane acetate
364782-34-3 Downstream products
-
226256-56-0

cinacalcet
-
1271930-15-4
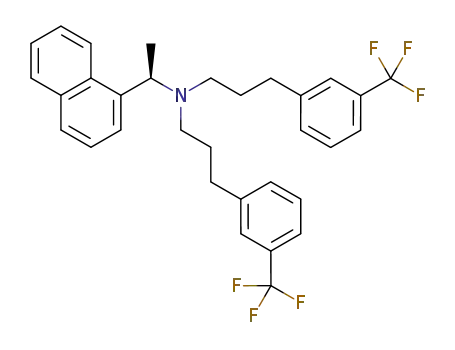
(R)-(1-naphthalen-1-ylethyl)-N,N-bis[3-(3-trifluoromethylphenyl)propyl]amine
-
1301700-82-2
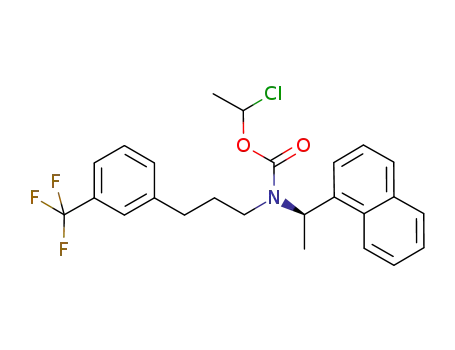
1-chloroethyl carbamate cinacalcet
Relevant Products
-
Selumetinib
CAS:606143-52-6
-
3-Amino-4-methylbenzoic acid ethyl ester
CAS:41191-92-8