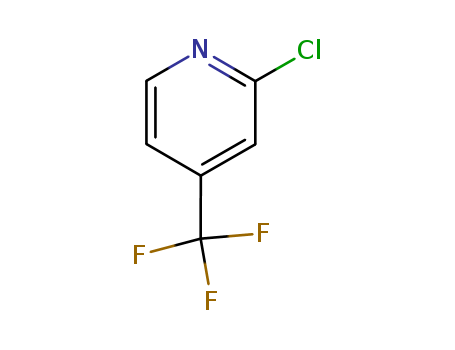
81565-18-6
- Product Name:2-Chloro-4-(trifluoromethyl)pyridine
- Molecular Formula:C6H3ClF3N
- Purity:99%
- Molecular Weight:181.545
Product Details
Grade Factory Supply High Purity Buy High 2-Chloro-4-(trifluoromethyl)pyridine 81565-18-6 Customized Supply
- Molecular Formula:C6H3ClF3N
- Molecular Weight:181.545
- Appearance/Colour:Light yellow liquid
- Vapor Pressure:0.0688mmHg at 25°C
- Melting Point:-19 °C
- Refractive Index:n20/D 1.4490(lit.)
- Boiling Point:172.5 °C at 760 mmHg
- PKA:-1.34±0.10(Predicted)
- Flash Point:58.1 °C
- PSA:12.89000
- Density:1.417 g/cm3
- LogP:2.75380
2-Chloro-4-(trifluoromethyl)pyridine(Cas 81565-18-6) Usage
|
Chemical Properties |
Light yellow liquid |
|
Uses |
2-Chloro-4-(trifluoromethyl)pyridine may be used in the synthesis of:4,4′-bis( trifluoromethyl)-2,2′-bipyridine4-(trifluoromethyl)pyridine1,3-bis(4-(trifluoromethyl)pyridin-2-yl)benzene |
|
General Description |
2-Chloro-4-(trifluoromethyl)pyridine can be synthesized from 2-chloro-4-iodopyridine. |
InChI:InChI=1/C11H11F3O2/c1-2-16-10(15)7-8-5-3-4-6-9(8)11(12,13)14/h3-6H,2,7H2,1H3
81565-18-6 Relevant articles
SUBSTITUTED AZOLE DIONE COMPOUNDS WITH ANTIVIRAL ACTIVITY
-
Paragraph 00363; 00405-00406, (2021/10/02)
Provided herein are methods of using sub...
PREPARATION OF SULFONAMIDE HERBICIDE PROCESS INTERMEDIATES
-
Paragraph 0051, (2020/07/15)
Improved methods for preparing chemical ...
Method for producing 2-chloro-4-trifluoromethylpyridine
-
Paragraph 0025; 0029, (2017/09/19)
The invention discloses a method for pro...
Trifluoromethylation of (hetero)aryl iodides and bromides with copper(i) chlorodifluoroacetate complexes
Lin, Xiaoxi,Li, Zhengyu,Han, Xiaoyan,Weng, Zhiqiang
, p. 75465 - 75469 (2016/08/24)
A new copper-mediated trifluoromethylati...
81565-18-6 Process route
-
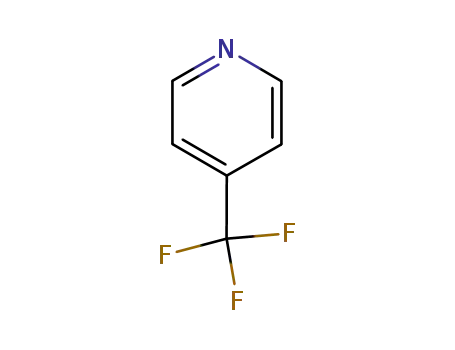
- 3796-24-5
4-trifluoromethylpyridine

-
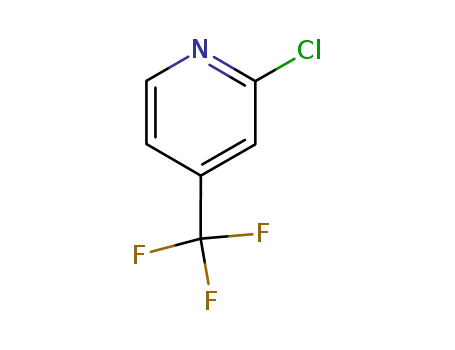
- 81565-18-6
2-chloro-4-trifluoromethyl pyridine
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1: 73 percent / MCPBA / CH2Cl2 / 10 h / 20 °C
2: 42 percent / SOCl2 / 3 h / Heating
With thionyl chloride; 3-chloro-benzenecarboperoxoic acid; In dichloromethane;
|
|
|
4-trifluoromethylpyridine; With 3-chloro-benzenecarboperoxoic acid; In ethyl acetate;
With trichlorophosphate;
|
|
|
Multi-step reaction with 2 steps
1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 10 h / 20 °C
2: thionyl chloride / Reflux
With thionyl chloride; 3-chloro-benzenecarboperoxoic acid; In dichloromethane;
|
|
|
Multi-step reaction with 2 steps
1: urea hydrogen peroxide adduct; trifluoroacetic anhydride / dichloromethane / 0 °C
2: trichlorophosphate / 90 °C
With urea hydrogen peroxide adduct; trifluoroacetic anhydride; trichlorophosphate; In dichloromethane;
|
|
|
Multi-step reaction with 2 steps
1: urea hydrogen peroxide adduct; pyridine; trifluoroacetic acid / dichloromethane / 0 °C
2: trichlorophosphate / 90 °C
With pyridine; urea hydrogen peroxide adduct; trifluoroacetic acid; trichlorophosphate; In dichloromethane;
|
-
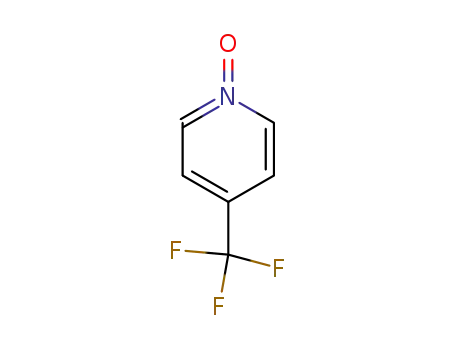
- 22253-59-4
1-oxido-4-(trifluoromethyl)pyridin-1-ium

-

- 81565-18-6
2-chloro-4-trifluoromethyl pyridine
| Conditions | Yield |
|---|---|
|
With thionyl chloride; Reflux;
|
62% |
|
With thionyl chloride; for 3h; Heating;
|
42% |
|
With trichlorophosphate; at 90 ℃;
|
|
|
With trichlorophosphate; at 90 ℃;
|
300 mg |
81565-18-6 Upstream products
-
22253-59-4

1-oxido-4-(trifluoromethyl)pyridin-1-ium
-
50650-59-4
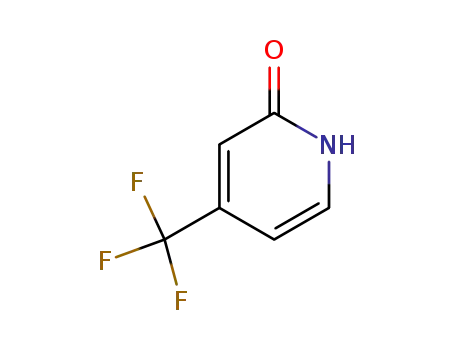
4-(trifluoromethyl)-2-pyridone
-
153034-86-7
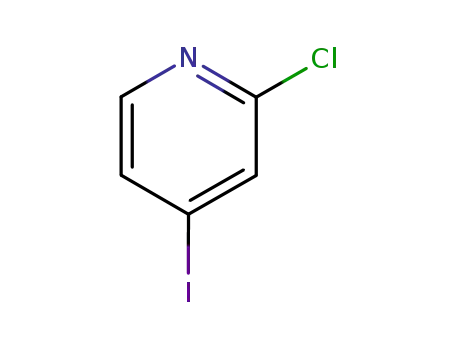
2-chloro-4-iodopyridine
-
81290-20-2
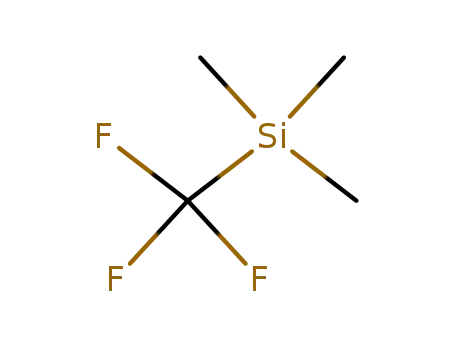
(trifluoromethyl)trimethylsilane
81565-18-6 Downstream products
-
121307-79-7
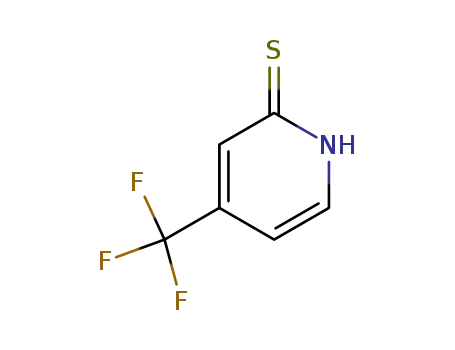
4-trifluoromethylpyridine-2(1H)-thione
-
89570-84-3
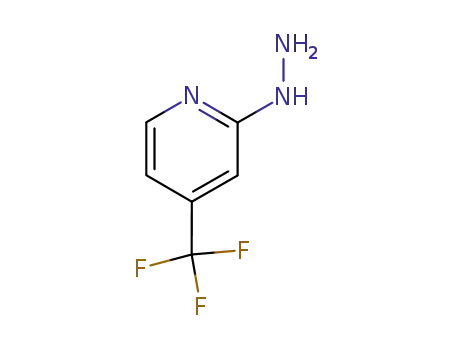
2-hydrazinyl-4-(trifluoromethyl)pyridine
-
142946-79-0
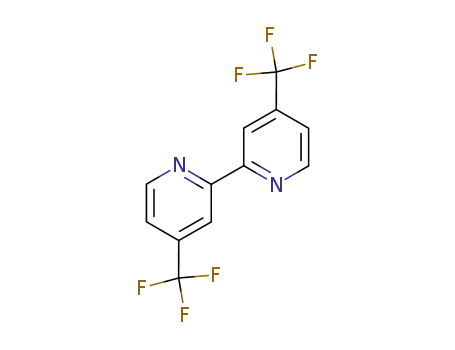
4,4′-bis-(trifluoromethyl)-2,2′-bipyridine
-
106447-97-6
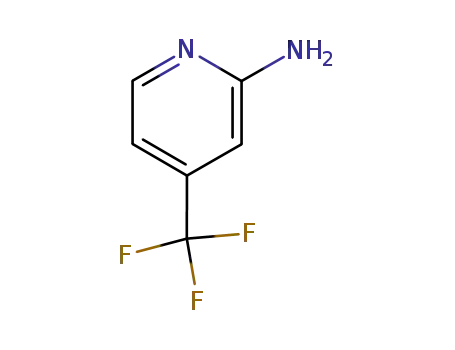
2-amino-4-(trifluoromethyl)pyridine
Relevant Products
-
Gefitinib int
CAS:184475-35-2
-
4-Chloro-N-methylpicolinamide
CAS:220000-87-3
-
4-(4-Aminophenoxy)-N-methylpicolinamide
CAS:284462-37-9