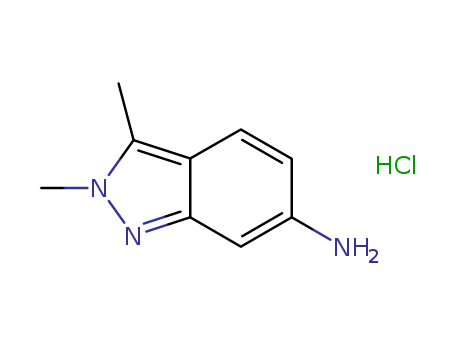
635702-60-2
- Product Name:2,3-dimethyl-2H-indazol-6-amine hydrochloride
- Molecular Formula:C9H11N3.HCl
- Purity:99%
- Molecular Weight:197.667
Product Details
Reputable Manufacturer Supply Reliable Quality 2,3-dimethyl-2H-indazol-6-amine hydrochloride 635702-60-2 Customized Supply
- Molecular Formula:C9H11N3.HCl
- Molecular Weight:197.667
- PSA:43.84000
- LogP:2.84710
2,3-dimethyl-2H-indazol-6-amine hydrochloride(Cas 635702-60-2) Usage
InChI:InChI=1/C9H11N3.ClH/c1-6-8-4-3-7(10)5-9(8)11-12(6)2;/h3-5H,10H2,1-2H3;1H
635702-60-2 Relevant articles
Method for preparing 2, 3-dimethyl-2H-indazole-6-benzylamine hydrochloride
-
Paragraph 0029; 0034-0035; 0039; 0040; 0044-0045; 0049, (2020/03/14)
The invention discloses a method for pre...
Pyrimidineamines as angiogenesis modulators
-
Paragraph 0178 - 0180, (2015/11/16)
Pyrimidine derivatives, which are useful...
Discovery of 5-[[4-[(2,3-dimethyl-2H-indazol-6-yl)methylamino]-2- pyrimidinyl]amino]-2-methyl-benzenesulfonamide (pazopanib), a novel and potent vascular endothelial growth factor receptor inhibitor
Harris, Philip A.,Boloor, Amogh,Cheung, Mui,Kumar, Rakesh,Crosby, Renae M.,Davis-Ward, Ronda G.,Epperly, Andrea H.,Hinkle, Kevin W.,Hunter III, Robert N.,Johnson, Jennifer H.,Knick, Victoria B.,Laudeman, Christopher P.,Luttrell, Deirdre K.,Mook, Robert A.,Nolte, Robert T.,Rudolph, Sharon K.,Szewczyk, Jerzy R.,Truesdale, Anne T.,Veal, James M.,Wang, Liping,Stafford, Jeffrey A.
experimental part, p. 4632 - 4640 (2009/06/18)
Inhibition of the vascular endothelial g...
Treatment Method
-
Page/Page column 8-9, (2009/01/20)
The present invention is directed to met...
635702-60-2 Process route
-
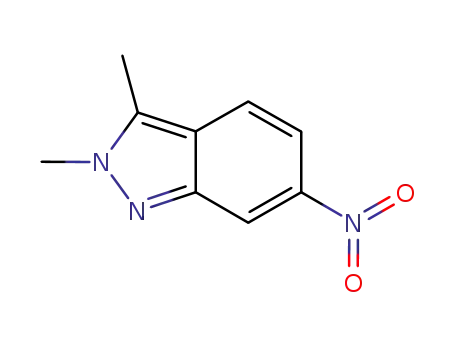
- 444731-73-1
2,3?dimethyl?6?nitro?2H?indazole

-
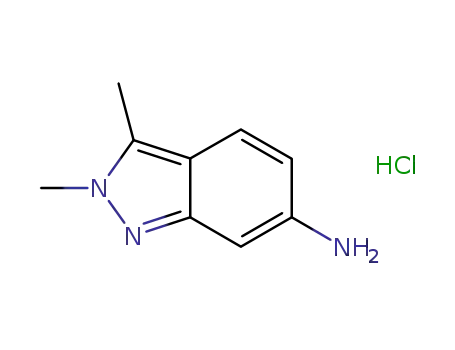
- 635702-60-2
2,3-dimethyl-2H-indazole-6-amine hydrochloride
| Conditions | Yield |
|---|---|
|
With hydrogenchloride; tin(ll) chloride; In diethylene glycol dimethyl ether; water; at 0 ℃; for 0.583333h;
|
96% |
|
With hydrogenchloride; tin(ll) chloride; In diethylene glycol dimethyl ether; water; at 0 ℃; for 0.583333h;
|
95% |
|
With hydrogenchloride; tin(ll) chloride; In diethylene glycol dimethyl ether; water; at 0 ℃;
|
95% |
|
With tin(ll) chloride; hydrogenchloride; In diethylene glycol dimethyl ether; water; at 0 ℃; for 0.583333h;
|
95% |
|
With hydrogenchloride; tin(ll) chloride; In diethylene glycol dimethyl ether; water; at 0 ℃; for 0.583333h; Inert atmosphere; Cooling with ice;
|
95% |
|
With hydrogenchloride; tin(ll) chloride; In diethylene glycol dimethyl ether; water; at 0 ℃; for 0.583333h;
|
95% |
|
With hydrogenchloride; tin(ll) chloride; In diethylene glycol dimethyl ether; water; at 0 ℃; for 0.583333h;
|
95% |
|
With hydrogenchloride; tin(ll) chloride; In diethylene glycol dimethyl ether; water; at 0 ℃; for 0.583333h;
|
95% |
|
With hydrogenchloride; tin(ll) chloride; In ethanol; water; at 0 ℃;
|
95% |
-
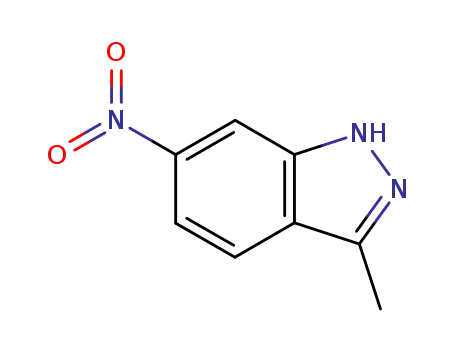
- 6494-19-5
3-methyl-6-nitro-1H-indazole

-

- 635702-60-2
2,3-dimethyl-2H-indazole-6-amine hydrochloride
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1.1: boron trifluoride diethyl etherate / dichloromethane / 0.25 h / -30 - 0 °C
1.2: 17.25 h / -70 - 20 °C
2.1: hydrogenchloride; tin(ll) chloride / diethylene glycol dimethyl ether; water / 0.58 h / 0 °C
With hydrogenchloride; boron trifluoride diethyl etherate; tin(ll) chloride; In dichloromethane; diethylene glycol dimethyl ether; water;
|
|
|
Multi-step reaction with 2 steps
1: sulfuric acid / dimethyl sulfoxide / 72 h / 50 °C
2: hydrogenchloride; tin(ll) chloride / diethylene glycol dimethyl ether; water / 0.58 h / 0 °C
With hydrogenchloride; sulfuric acid; tin(ll) chloride; In diethylene glycol dimethyl ether; water; dimethyl sulfoxide;
|
|
|
Multi-step reaction with 2 steps
1: acetone / 3 h / 20 °C
2: hydrogenchloride; tin(ll) chloride / diethylene glycol dimethyl ether; water / 0.58 h / 0 °C
With hydrogenchloride; tin(ll) chloride; In diethylene glycol dimethyl ether; water; acetone;
|
|
|
Multi-step reaction with 2 steps
1.1: boron trifluoride diethyl etherate / dichloromethane / 0.28 h / -30 - 0 °C
1.2: 17.25 h / -70 - 20 °C
2.1: hydrogenchloride; tin(ll) chloride / diethylene glycol dimethyl ether; water / 0.58 h / 0 °C
With hydrogenchloride; boron trifluoride diethyl etherate; tin(ll) chloride; In dichloromethane; diethylene glycol dimethyl ether; water;
|
|
|
Multi-step reaction with 2 steps
1: boron trifluoride diethyl etherate / dichloromethane / 17.53 h / -70 - 20 °C
2: hydrogenchloride; tin(ll) chloride / diethylene glycol dimethyl ether; water / 0.58 h / 0 °C
With hydrogenchloride; boron trifluoride diethyl etherate; tin(ll) chloride; In dichloromethane; diethylene glycol dimethyl ether; water;
|
635702-60-2 Upstream products
-
444731-73-1

2,3?dimethyl?6?nitro?2H?indazole
-
6494-19-5

3-methyl-6-nitro-1H-indazole
-
635702-59-9
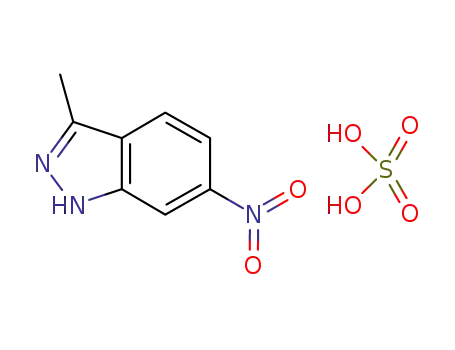
3-methyl-6-nitro-1H-indazole sulfate
-
20191-74-6
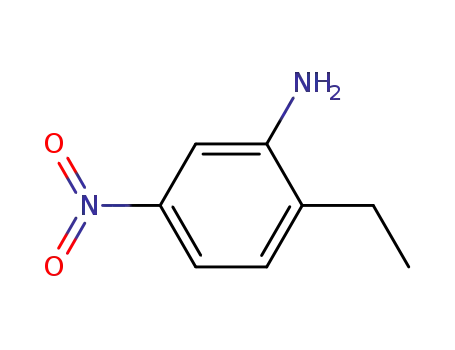
2-ethyl-5-nitroaniline
635702-60-2 Downstream products
-
444731-74-2
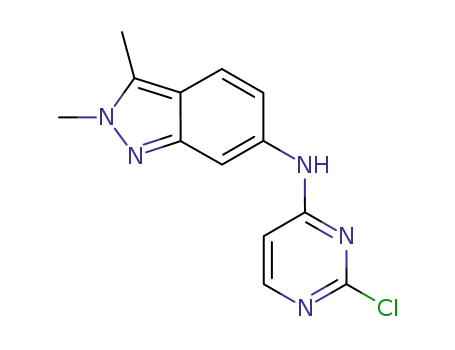
N-(2-chloropyrimidin-4-yl)-2,3-dimethyl-2H-indazol-6-amine
-
1226499-98-4
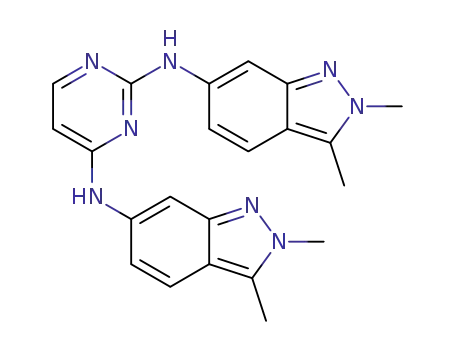
N2,N4-bis(2,3-dimethyl-2H-indazol-6-yl)-pyrimidine-2,4-diamine
Relevant Products
-
Sunitinib Malate int
CAS:341031-54-7
-
2-Hydroxy-6-(trifluoromethyl)puridine
CAS:34486-06-1