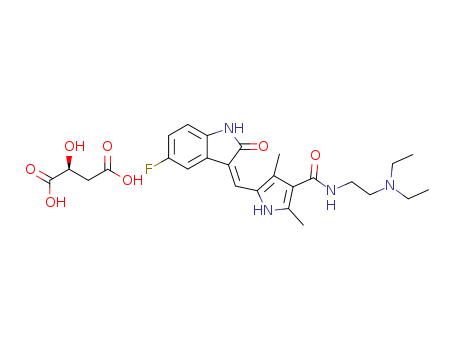
341031-54-7
- Product Name:Sunitinib Malate int
- Molecular Formula:C22H27FN4O2.C4H6O5
- Purity:99%
- Molecular Weight:532.569
Product Details
Trustworthy Manufacturer Supply Reliable Quality Sunitinib Malate int 341031-54-7 with Fast Delivery
- Molecular Formula:C22H27FN4O2.C4H6O5
- Molecular Weight:532.569
- Melting Point:189-191°C
- Boiling Point:572.1 °C at 760 mmHg
- Flash Point:299.8 °C
- PSA:172.06000
- Density:1.3600g/mLat 25°C(lit.)
- LogP:2.77040
Sunitinib malate(Cas 341031-54-7) Usage
|
Anticancer drug |
Sunitinib malate is a kind of novel oral multi-targeted anticancer drugs and belongs to multi-targeted tyrosine kinase inhibitor with its trade name being “suntent”. It was successfully developed by Pfizer Company and has dual anti-tumor effect. Moreover, it is the only therapeutic drug which can go beyond the 2-year survival period of advanced kidney cancer and plays a central role in the field of carcinoma and gastrointestinal stromal tumor therapeutic areas. It entered into market in February 2006 in the United States. This drug was the first anti-cancer drug which was approved by the US FDA and can simultaneously treat two diseases. Sunitinib exerts its therapeutic role through preventing the tumor cells from getting the blood and nutrients needed for growth. Clinical trials have showed that the drug can delay the growth rate of gastrointestinal stromal tumors, and can shrink the size of renal cell tumors. Sunitinib malate is the first novel targeted drug being capable of selectively targets drugs for a variety of new tyrosine kinase receptors. It combine two kinds of anti-tumor mechanisms including both stopping the formation of anti-angiogenic which is responsible for supplying blood to tumor and direct attack of tumor cells. It represents the advent of a new round of targeted therapies, being able to attack the tumor directly without the toxicity of the conventional chemotherapy. The indications of Sunitinib malate are as follows: 1, Patients who failed in the treatment with matinib mesylate or of intolerant gastrointestinal stromal tumors (GIST). 2, inoperable advanced renal cell carcinoma (RCC). 3, advanced pancreatic endocrine tumors. The above information is edited by the lookchem of Dai Xiongfeng. |
|
Uses |
Anti-cancer drugs |
|
Description |
Sunitinib is a small molecule inhibitor of receptor tyrosine kinases, including FLK1 (Ki = 9 nM), PDGFRβ (Ki = 8 nM), and FLT3. It is at least 10-fold selective for FLK1 and PDGFRβ over a variety of tyrosine kinases in a panel, including EGFR, Cdk2, Met, IGFR-1, Abl, and Src. Sunitinib inhibits VEGF-dependent FLK1 and PDGF-dependent PDGFRβ phosphorylation (IC50s = 10 and 10 nM, respectively) as well as phosphorylation of FLT3 and FLT3 carrying the activating internal tandem duplication mutation (FLT3-ITD; IC50s = 250 and 50 nM, respectively). It decreases VEGF- and FGF-induced proliferation of human umbilical vein endothelial cells (HUVECs; IC50s = 30 and 700 nM, respectively) and reduces tumor growth in a variety of mouse xenograft models when administered at doses ranging from 20 to 80 mg/kg per day. Formulations containing sunitinib have been used in the treatment of gastrointestinal stromal tumors and metastatic renal cell carcinoma. |
|
Chemical Properties |
Yellow Solid |
|
Brand name |
(Pfizer). |
|
General Description |
Sunitinib is available in 12.5-, 25-, and 50-mg capsules fororal administration for the treatment of advanced RCC andGIST upon the failure of imatinib. The agent is a multikinaseinhibitor and has been shown to inhibit PDGF-R,VEGF-R, Kit, RET, and the colony-stimulating factor receptor(CSR-1R). The result of this spectrum of activity isa slowing of tumor progression and inhibition of angiogenesisboth of which are useful in the highly vascularizedcancers, RCC and GIST. The median TTP (time to tumorprogression) was 27.3 weeks for sunitinib and 6.4 weeks with a median time of 24.1 weeks for sunitinib and 6 weeksfor placebo. The agent is well absorbed upon oral administration,and both the parent and major metabolite are highly(90%–95%) protein bound. Metabolism is mediated primarilyby CYP3A4 to give the N-desethyl derivative, which isthe major metabolite (23%–37%), equally active with theparent and undergoes further metabolism by CYP3A4. Theterminal elimination half-life for the parent and N-desethylderivative are 40 to 60 hours and 80 to 110 hours, respectively.Elimination occurs primarily via the feces. Commonadverse effects of sunitinib include fatigue, diarrhea, yellowskin discoloration, anorexia, nausea, and mucositis.Mild myelosuppression has been reported in patients withGIST including neutropenia, lymphopenia, thrombocytopenia,and anemia. There have been reports of cardiotoxicityincluding decreases in left ventricular ejectionfraction, which occurred in 11% of patients during a GISTstudy. |
|
Biological Activity |
Potent, ATP-competitive VEGFR, PDGFR β and KIT inhibitor (K i values are 2, 9, 17, 8 and 4 nM for VEGFR -1, -2, -3, PDGFR β and KIT respectively). Also inhibits cellular receptor phosphorylation of FLT3, RET and CSF-1R. Exhibits antiangiogenic and antitumor activity in multiple xenograft models. |
|
Biochem/physiol Actions |
Sunitinib has greater bioavailability and potency compared to other inhibitors. It prevents angiogenesis. |
InChI:InChI=1/C23H30N4O2.C5H8O5/c1-6-27(7-2)11-10-24-23(29)21-15(4)20(25-16(21)5)13-18-17-12-14(3)8-9-19(17)26-22(18)28;6-3(5(9)10)1-2-4(7)8/h8-9,12-13,25H,6-7,10-11H2,1-5H3,(H,24,29)(H,26,28);3,6H,1-2H2,(H,7,8)(H,9,10)/b18-13-;/t;3-/m.0/s1
341031-54-7 Relevant articles
A high-purity malic acid lin's preparation method
-
Paragraph 0042; 0043, (2019/04/04)
The invention relates to a high-purity m...
High-purity L-sunitinib malate preparation method
-
Paragraph 0048; 0050, (2017/08/28)
The present invention discloses a high-p...
PROCESS FOR THE PERPARATION OF SUNITINIB AND ITS ACID ADDITION SALTS THEREOF
-
Paragraph 0115-0120, (2015/02/18)
The present invention relates to an impr...
PROCESSES FOR THE PREPARATION OF 3-(PYRROL-2-YL)METHYLENE)-2-PYRROLONES USING 2-SILYLOXY-PYRROLES
-
Paragraph 0060; 0061, (2013/07/31)
The present invention provides for synth...
341031-54-7 Process route
-
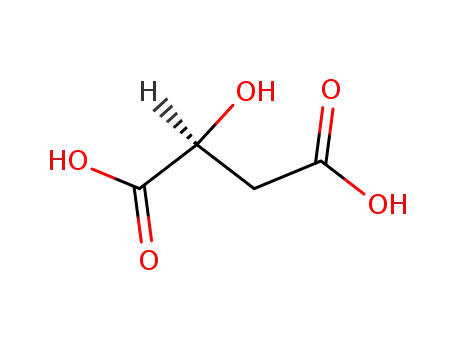
- 97-67-6,78644-42-5
(S)-Malic acid

-
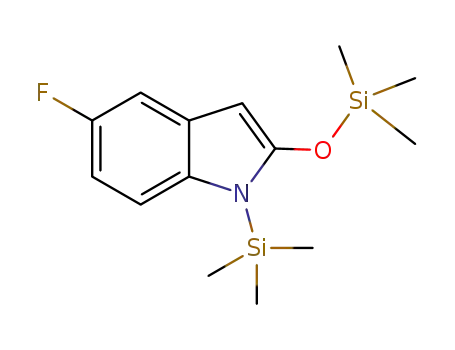
- 1374685-40-1
5-fluoro-1-(trimethylsilyl)-2-(trimethylsilyloxy)-1H-indole

-
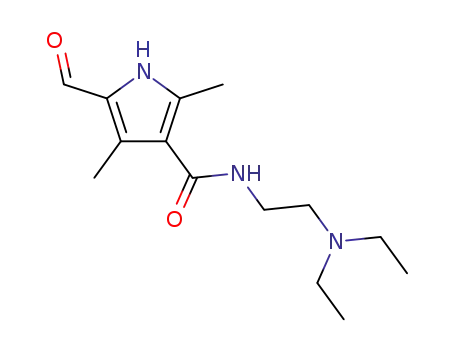
- 356068-86-5
N-(2-(diethylamino)ethyl)-5-formyl-2,4-dimethyl-1H-pyrrole-3-carboxamide

-
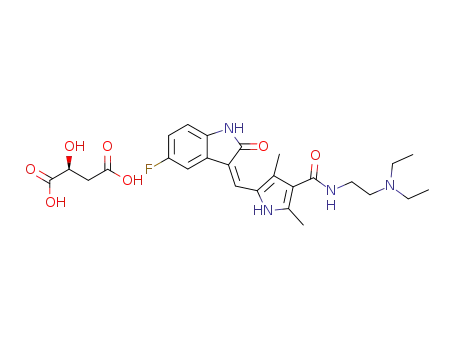
- 341031-54-7
sunitinib malate
| Conditions | Yield |
|---|---|
|
5-fluoro-1-(trimethylsilyl)-2-(trimethylsilyloxy)-1H-indole; N-(2-(diethylamino)ethyl)-5-formyl-2,4-dimethyl-1H-pyrrole-3-carboxamide; trimethylsilyl trifluoromethanesulfonate; In acetonitrile; for 13h; Reflux;
(S)-Malic acid; In methanol; acetonitrile; at 20 ℃; for 22h;
|
70% |
|
5-fluoro-1-(trimethylsilyl)-2-(trimethylsilyloxy)-1H-indole; N-(2-(diethylamino)ethyl)-5-formyl-2,4-dimethyl-1H-pyrrole-3-carboxamide; With trimethylsilyl trifluoromethanesulfonate; In acetonitrile; for 13h; Reflux;
(S)-Malic acid; In methanol; for 24h;
|
|
|
5-fluoro-1-(trimethylsilyl)-2-(trimethylsilyloxy)-1H-indole; N-(2-(diethylamino)ethyl)-5-formyl-2,4-dimethyl-1H-pyrrole-3-carboxamide; With trimethylsilyl trifluoromethanesulfonate; In acetonitrile; for 9.5h; Reflux;
(S)-Malic acid; In methanol; for 24h;
|
-
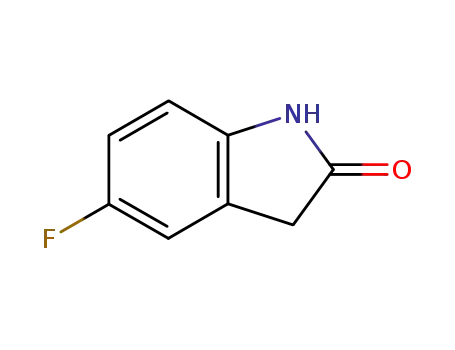
- 56341-41-4
5-fluoroindol-2(3H)-one

-
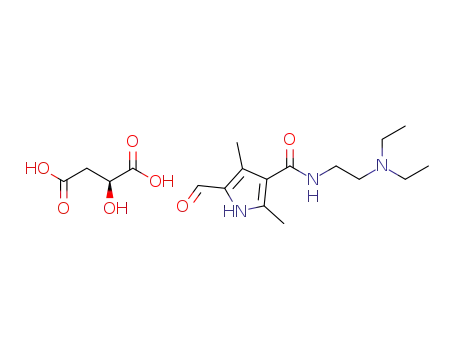
- 1200435-83-1
L-(-)-malic acid of 5-formyl-2,4-dimethyl-1H-pyrrole-3-carboxylic acid (2-diethylaminoethyl)amide

-

- 341031-54-7
sunitinib malate
| Conditions | Yield |
|---|---|
|
With pyrrolidine; In butan-1-ol; at 20 ℃; Reflux;
|
75.1% |
341031-54-7 Upstream products
-
97-67-6

(S)-Malic acid
-
557795-19-4
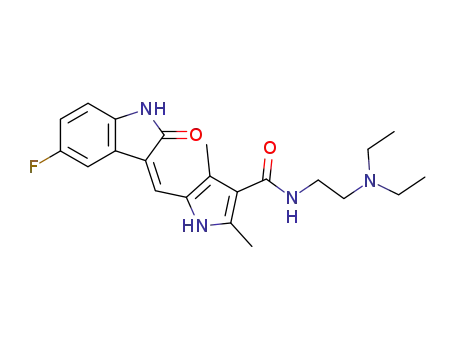
sunitinib
-
56341-41-4

5-fluoroindol-2(3H)-one
-
356068-86-5

N-(2-(diethylamino)ethyl)-5-formyl-2,4-dimethyl-1H-pyrrole-3-carboxamide
341031-54-7 Downstream products
-
557795-19-4

sunitinib
Relevant Products
-
2-ethyl-5-nitroaniline
CAS:20191-74-6
-
2,3-dimethyl-2H-indazol-6-amine hydrochloride
CAS:635702-60-2