755037-03-7
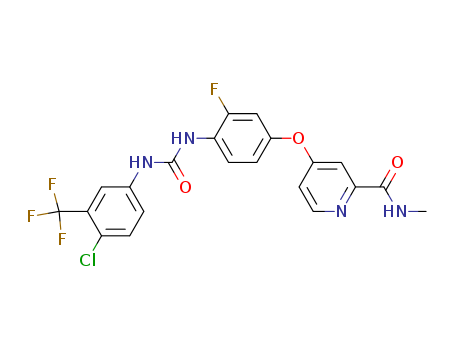
- Product Name:Regorafenib int
- Molecular Formula:C21H15ClF4N4O3
- Purity:99%
- Molecular Weight:482.822
Product Details
Reputable Manufacturer Supply High Purity 99% Regorafenib int 755037-03-7 with Low Price
- Molecular Formula:C21H15ClF4N4O3
- Molecular Weight:482.822
- Melting Point:206.0 to 210.0 °C
- Boiling Point:513.4 °C at 760 mmHg
- PKA:12.04±0.70(Predicted)
- Flash Point:264.3 °C
- PSA:92.35000
- Density:1.491 g/cm3
- LogP:6.22570
755037-03-7 Relevant articles
Synthesis method of regorafenib
-
Paragraph 0029-0031, (2021/04/21)
The invention provides a synthesis metho...
Interrupted aza-Wittig reactions using iminophosphoranes to synthesize 11C-carbonyls
Ismailani, Uzair S.,Munch, Maxime,Mair, Braeden A.,Rotstein, Benjamin H.
supporting information, p. 5266 - 5269 (2021/06/06)
A direct CO2-fixation methodology couple...
Preparation method of regorafenib
-
Paragraph 0025-0040, (2021/05/29)
The invention relates to a preparation m...
Regorafenib analogues and their ferrocenic counterparts: Synthesis and biological evaluation
Wilde, Myron,Arzur, Danielle,Baratte, Blandine,Lefebvre, Dorian,Robert, Thomas,Roisnel, Thierry,Le Jossic-Corcos, Catherine,Bach, Stéphane,Corcos, Laurent,Erb, William
supporting information, p. 19723 - 19733 (2020/12/04)
Approved by the FDA in 2012, regorafenib...
755037-03-7 Process route
-
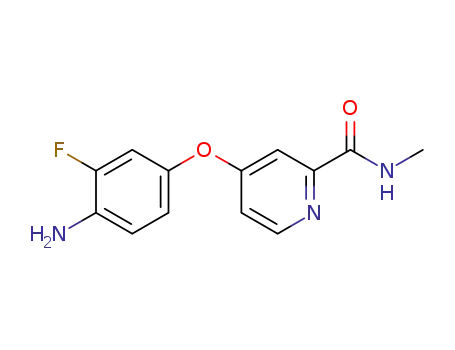
- 757251-39-1
4-(4-amino-3-fluorophenoxy)pyridine-2-carboxylic acid methyl amide

-
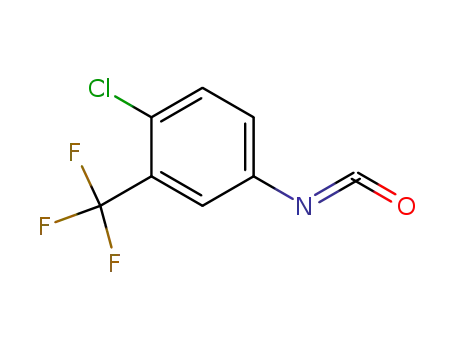
- 327-78-6
4-chloro-3-(trifluoromethyl)phenyl isocyanate

-
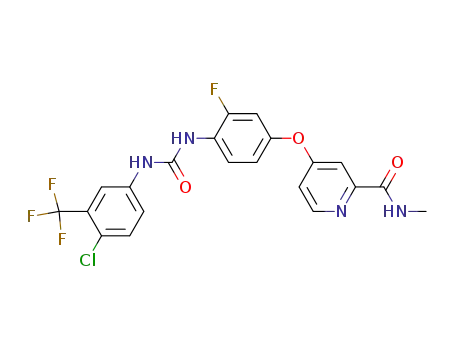
- 755037-03-7
regorafenib
| Conditions | Yield |
|---|---|
|
In ethyl acetate; at 20 ℃; for 1.5h;
|
94.5% |
|
In dichloromethane; at 0 - 20 ℃; for 16h; Inert atmosphere;
|
90% |
|
In dichloromethane; for 24h;
|
87% |
|
In acetone; at 40 ℃; for 5h;
|
85% |
|
In tetrahydrofuran; toluene; at 27 ℃; for 4.5h;
|
74.9% |
|
In tetrahydrofuran; at 30 ℃; for 12h;
|
73.46% |
|
In dichloromethane; at 0 - 25 ℃; for 3h;
|
67.2% |
|
In toluene; at 20 ℃; for 72h;
|
47% |
|
In toluene; at 20 ℃; for 72h;
|
47% |
|
In toluene; at 20 ℃; for 72h;
|
47% |
|
In toluene; at 20 ℃; for 72h;
|
47% |
|
In toluene; at 20 ℃; for 72h;
|
47% |
|
In ethyl acetate; at 20 - 45 ℃; for 1.5h;
|
|
|
4-(4-amino-3-fluorophenoxy)pyridine-2-carboxylic acid methyl amide; 4-chloro-3-(trifluoromethyl)phenyl isocyanate; In tetrahydrofuran; toluene; at 20 ℃; for 4.5h;
With acetyl chloride; In tetrahydrofuran; methanol; toluene; for 2.25h;
|
|
|
In dichloromethane; at 0 - 5 ℃; for 4h; Inert atmosphere;
|
350 g |
|
In acetone; at 25 - 30 ℃;
|
5.5 g |
|
In ethyl acetate; at 20 ℃; for 0.5h;
|
|
|
4-(4-amino-3-fluorophenoxy)pyridine-2-carboxylic acid methyl amide; 4-chloro-3-(trifluoromethyl)phenyl isocyanate; In tetrahydrofuran; at 25 ℃; for 4h; Inert atmosphere;
With hydrogenchloride; In water; at 25 ℃; for 4h;
|
|
|
4-(4-amino-3-fluorophenoxy)pyridine-2-carboxylic acid methyl amide; In dichloromethane; at 25 - 30 ℃; Inert atmosphere;
4-chloro-3-(trifluoromethyl)phenyl isocyanate; In dichloromethane; at 0 - 30 ℃;
|
|
|
In tetrahydrofuran; at 20 ℃; for 5h;
|
86.7 g |
|
4-(4-amino-3-fluorophenoxy)pyridine-2-carboxylic acid methyl amide; 4-chloro-3-(trifluoromethyl)phenyl isocyanate; With acetic acid; In tetrahydrofuran; toluene; at 20 ℃; for 4.5h;
With acetyl chloride; In tetrahydrofuran; methanol; for 2.25h;
With water; sodium hydroxide; In acetone; at 40 ℃; for 0.5h;
|
31.5 g |
|
In dichloromethane; ethyl acetate; at 20 ℃; for 18h; Inert atmosphere; Schlenk technique;
|
169 mg |
-

- 757251-39-1
4-(4-amino-3-fluorophenoxy)pyridine-2-carboxylic acid methyl amide

-
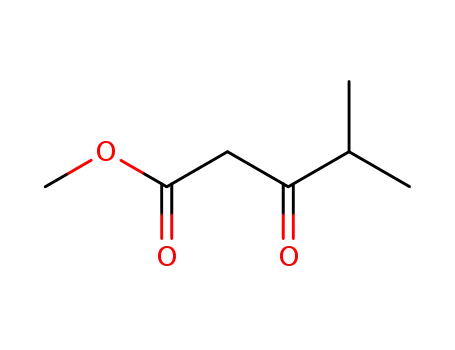
- 42558-54-3
Methyl 4-methyl-3-oxopentanoate

-
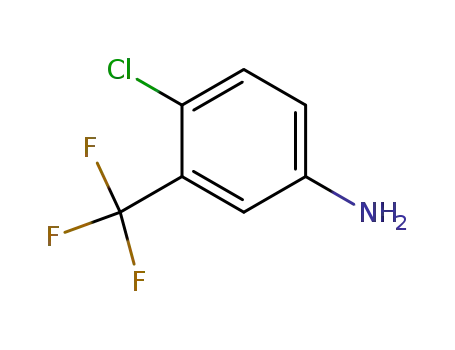
- 320-51-4
4-chloro-3-trifluoromethyl-aniline

-

- 755037-03-7
regorafenib
| Conditions | Yield |
|---|---|
|
With dmap; In N,N-dimethyl-formamide; at 140 ℃; for 4h; Temperature; Green chemistry;
|
96.8% |
755037-03-7 Upstream products
-
757251-39-1

4-(4-amino-3-fluorophenoxy)pyridine-2-carboxylic acid methyl amide
-
327-78-6

4-chloro-3-(trifluoromethyl)phenyl isocyanate
-
399-95-1
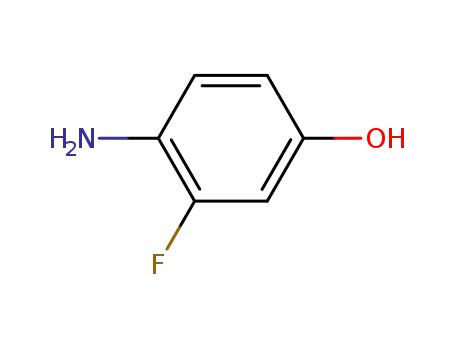
4-amino-3-fluorophenol
-
1338722-52-3
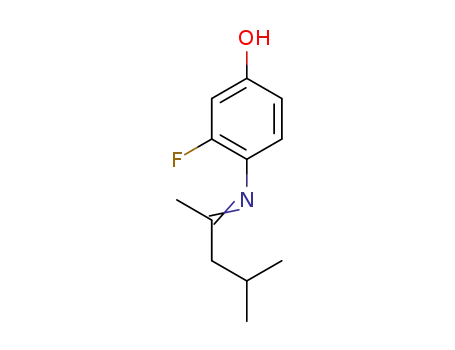
C12H16FNO
755037-03-7 Downstream products
-
835621-08-4
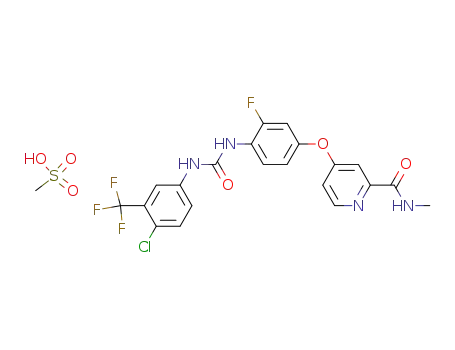
4-{4-[3-(4-chloro-3-trifluoromethylphenyl)-ureido]-3-fluorophenoxy}-pyridine-2-carboxylic acid methylamide mesylate
-
835621-09-5
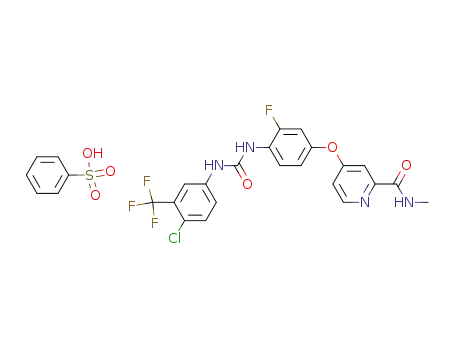
4-{4-[3-(4-chloro-3-trifluoromethylphenyl)-ureido]-3-fluorophenoxy}-pyridine-2-carboxylic acid methylamide phenylsulfonate
-
835621-07-3
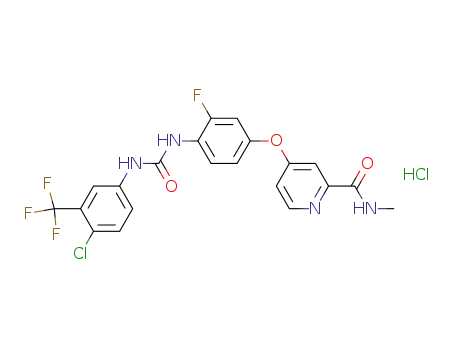
4-{4-[3-(4-chloro-3-trifluoromethylphenyl)-ureido]-3-fluorophenoxy}-pyridine-2-carboxylic acid methylamide hydrochloride
-
1019206-88-2
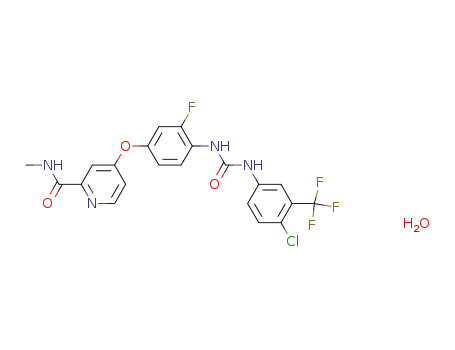
4-[4({[4-chloro-3-(trifluoromethyl)phenyl]carbamoyl}amino)-3-fluorophenoxy]-N-methylpyridine-2-carboxamide monohydrate
Relevant Products
-
Gefitinib int
CAS:184475-35-2
-
2-Amino-5-methylpyridine
CAS:1603-41-4
-
Regorafenib (Hydrochloride) int
CAS:835621-07-3