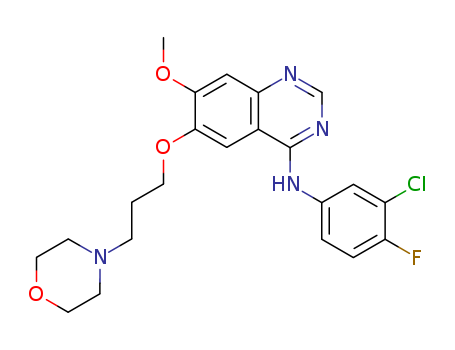
444731-75-3
- Product Name:N-(2-chloropyriMidin-4-yl)-N,2,3-triMethyl-2H-indazol-6-aMine
- Molecular Formula:C14H14ClN5
- Purity:99%
- Molecular Weight:287.752
Product Details
Factory Supply Sells Quality N-(2-chloropyriMidin-4-yl)-N,2,3-triMethyl-2H-indazol-6-aMine 444731-75-3 In Stock
- Molecular Formula:C14H14ClN5
- Molecular Weight:287.752
- Vapor Pressure:0mmHg at 25°C
- Melting Point:167-173℃
- Refractive Index:1.673
- Boiling Point:524.447 °C at 760 mmHg
- PKA:2.82±0.30(Predicted)
- Flash Point:270.976 °C
- PSA:46.84000
- Density:1.339 g/cm3
- LogP:3.09300
N-(2-chloropyriMidin-4-yl)-N,2,3-triMethyl-2H-indazol-6-aMine(Cas 444731-75-3) Usage
|
Uses |
N-(2-Chloro-4-pyrimidinyl)-N,2,3-trimethyl-2H-indazol-6-amine is an impurity of Pazopanib (P210925), an oral angiogenesis inhibitor targeting VEGFR and PDGFR. |
InChI:InChI=1/C14H14ClN5/c1-9-11-5-4-10(8-12(11)18-20(9)3)19(2)13-6-7-16-14(15)17-13/h4-8H,1-3H3
444731-75-3 Relevant articles
A NOVEL PROCESS FOR PREPARATION OF PAZOPANIB HYDROCHLORIDE
-
Page/Page column 11, (2021/08/20)
The present invention relates to a new p...
Preparation method of pazopanib intermediate
-
Paragraph 0181-0184, (2021/03/24)
The invention provides a preparation met...
Process-Related Impurities of Pazopanib
Terentjeva, Svetlana,Muceniece, Dzintra,LūSis, Viesturs
, p. 2057 - 2068 (2019/08/20)
The main target of this study was to syn...
Green preparation method of palatinib hydrochloride (by machine translation)
-
Paragraph 0086-0094, (2019/08/01)
The invention belongs to the technical f...
444731-75-3 Process route
-
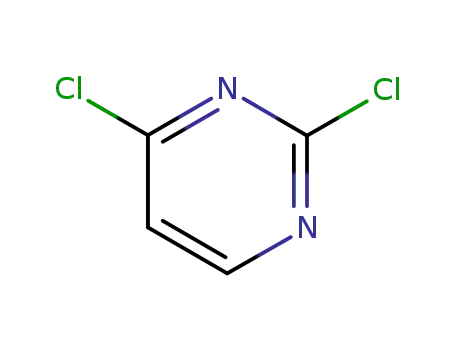
- 3934-20-1
2,6-Dichloropyrimidine

-
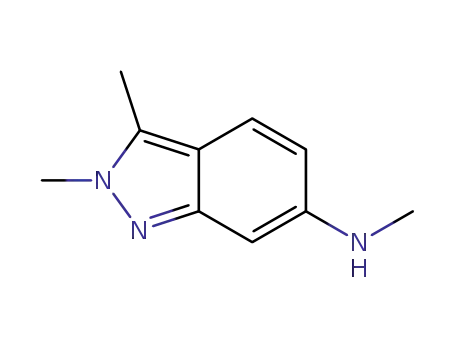
- 1376676-65-1
N,2,3-trimethyl-2H-indazole-6-amine

-
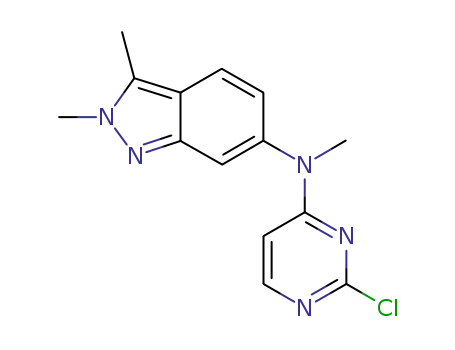
- 444731-75-3
N-(2-chloropyrimidin-4-yl)-N-methyl-2,3-dimethyl-2H-indazole-6-amine
| Conditions | Yield |
|---|---|
|
With sodium hydrogencarbonate; In water; N,N-dimethyl-formamide; at 85 ℃; for 3h;
|
97% |
|
With sodium carbonate; In N,N-dimethyl-formamide; at 100 ℃; for 3h; Inert atmosphere;
|
88.4% |
|
With sodium hydrogencarbonate; In tetrahydrofuran; ethanol; at 70 - 75 ℃; for 16h;
|
85% |
|
With sodium hydrogencarbonate; In tetrahydrofuran; ethanol; for 20h; Solvent; Reflux;
|
81.6% |
-
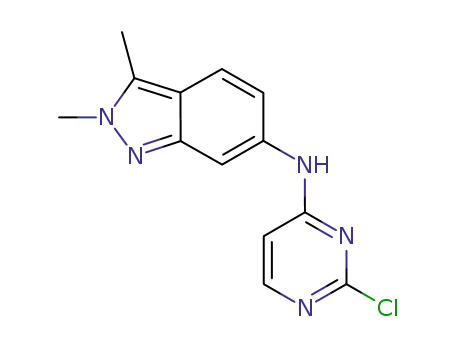
- 444731-74-2
N-(2-chloropyrimidin-4-yl)-2,3-dimethyl-2H-indazol-6-amine

-
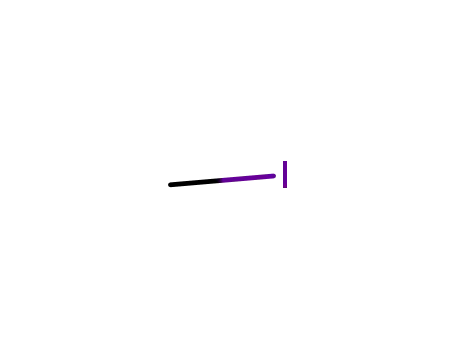
- 74-88-4
methyl iodide

-

- 444731-75-3
N-(2-chloropyrimidin-4-yl)-N-methyl-2,3-dimethyl-2H-indazole-6-amine
| Conditions | Yield |
|---|---|
|
N-(2-chloropyrimidin-4-yl)-2,3-dimethyl-2H-indazol-6-amine; With caesium carbonate; In DMF (N,N-dimethyl-formamide); at 20 - 25 ℃; for 0.166667h;
methyl iodide; In DMF (N,N-dimethyl-formamide); at 20 - 30 ℃; for 1 - 2h;
|
90.4% |
|
With caesium carbonate; In N,N-dimethyl-formamide; at 25 - 30 ℃; for 6h;
|
90.4% |
|
N-(2-chloropyrimidin-4-yl)-2,3-dimethyl-2H-indazol-6-amine; With caesium carbonate; In N,N-dimethyl-formamide; at 20 - 25 ℃; for 0.166667h;
methyl iodide; In N,N-dimethyl-formamide; at 20 - 30 ℃; for 1.16667 - 2.16667h; Product distribution / selectivity;
|
90.4% |
|
N-(2-chloropyrimidin-4-yl)-2,3-dimethyl-2H-indazol-6-amine; With caesium carbonate; In N,N-dimethyl-formamide; at 20 - 25 ℃; for 0.166667h;
methyl iodide; In water; N,N-dimethyl-formamide; at 20 - 40 ℃; for 1.33333h; Product distribution / selectivity;
|
90.4% |
|
With caesium carbonate; In N,N-dimethyl-formamide; at 20 ℃; for 5h;
|
89.1% |
|
With caesium carbonate; In N,N-dimethyl-formamide; at 20 ℃; for 2h;
|
85.7% |
|
With caesium carbonate; In N,N-dimethyl-formamide; at 20 ℃; Product distribution / selectivity;
|
83% |
|
With caesium carbonate; In N,N-dimethyl-formamide; at 20 ℃; for 16h;
|
83% |
|
With caesium carbonate; In DMF (N,N-dimethyl-formamide); at 20 - 30 ℃; for 1 - 2h;
|
83% |
|
With caesium carbonate; In N,N-dimethyl-formamide; at 20 - 30 ℃; Product distribution / selectivity;
|
83% |
|
With caesium carbonate; In N,N-dimethyl-formamide; at 20 ℃; Inert atmosphere;
|
83% |
|
With caesium carbonate; In N,N-dimethyl-formamide; at 20 ℃; Product distribution / selectivity;
|
83% |
|
With caesium carbonate; In N,N-dimethyl-formamide; at 20 - 30 ℃; Product distribution / selectivity;
|
83% |
|
With caesium carbonate; In N,N-dimethyl-formamide; at 20 ℃; Product distribution / selectivity;
|
83% |
|
N-(2-chloropyrimidin-4-yl)-2,3-dimethyl-2H-indazol-6-amine; With caesium carbonate; In N,N-dimethyl-formamide; at 20 ℃; for 0.333333h;
methyl iodide; In N,N-dimethyl-formamide; at 20 ℃; for 2h;
|
80% |
|
N-(2-chloropyrimidin-4-yl)-2,3-dimethyl-2H-indazol-6-amine; With caesium carbonate; In N,N-dimethyl-formamide; at 20 ℃; for 0.5h; Inert atmosphere;
methyl iodide; In N,N-dimethyl-formamide; at 20 ℃; for 2h; Inert atmosphere;
|
80% |
|
N-(2-chloropyrimidin-4-yl)-2,3-dimethyl-2H-indazol-6-amine; With caesium carbonate; In N,N-dimethyl-formamide; for 0.5h;
methyl iodide; In N,N-dimethyl-formamide; at 20 ℃; for 2h;
|
37% |
|
N-(2-chloropyrimidin-4-yl)-2,3-dimethyl-2H-indazol-6-amine; With caesium carbonate; In N,N-dimethyl-formamide; at 20 ℃; for 0.166667h;
methyl iodide; In N,N-dimethyl-formamide; at 20 ℃; for 24h;
|
31% |
444731-75-3 Upstream products
-
74-88-4

methyl iodide
-
444731-74-2

N-(2-chloropyrimidin-4-yl)-2,3-dimethyl-2H-indazol-6-amine
-
616-38-6
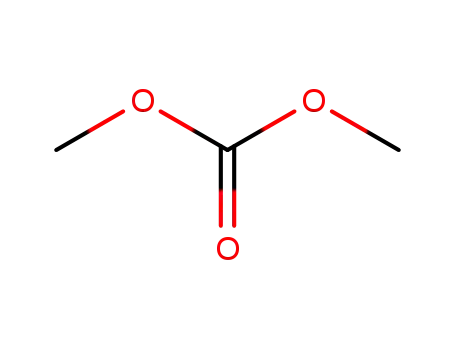
carbonic acid dimethyl ester
-
444731-66-2
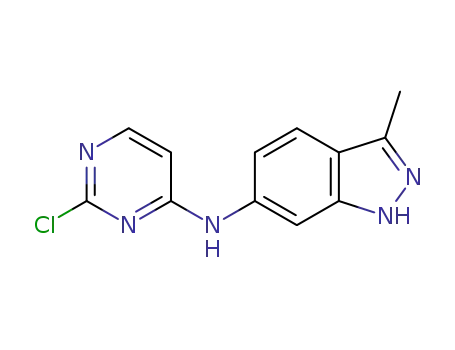
N-(2-chloro-4-pyrimidinyl)-N-(3-methyl-1H-indazol-6-yl)amine
444731-75-3 Downstream products
-
635702-64-6
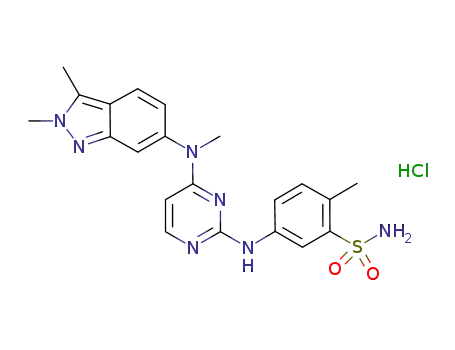
pazopanib hydrochloride
-
444731-52-6
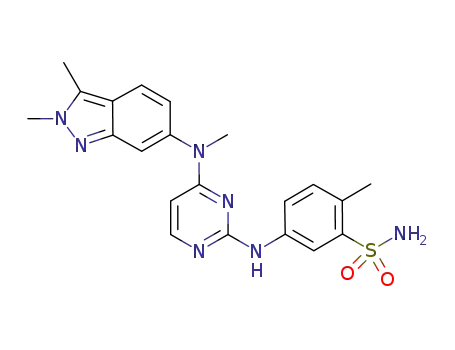
pazopanib
-
635702-64-6
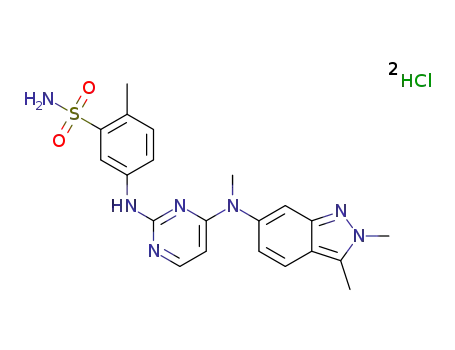
pazopanib dihydrochloride
-
1307297-53-5
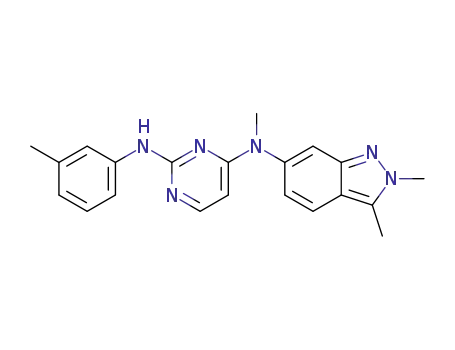
N4-(2,3-dimethyl-2H-indazol-6-yl)-N4-methyl-N2-m-tolylpyrimidine-2,4-diamine
Relevant Products
-
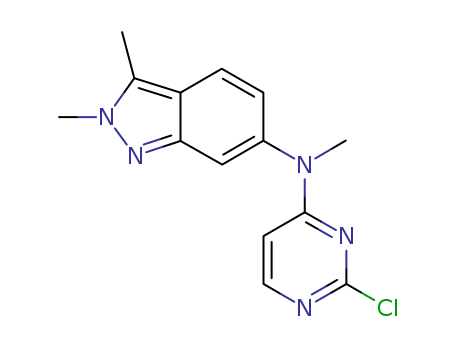
Gefitinib int
CAS:184475-35-2
-
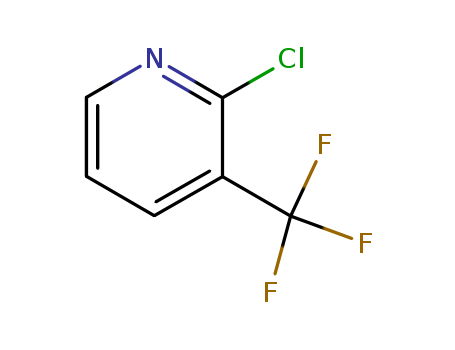
2-Chloro-3-(trifluoromethyl)pyridine
CAS:65753-47-1
-
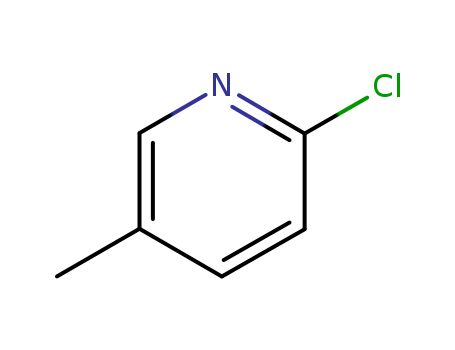
2-Chloro-5-methylpyridine
CAS:18368-64-4