444731-74-2
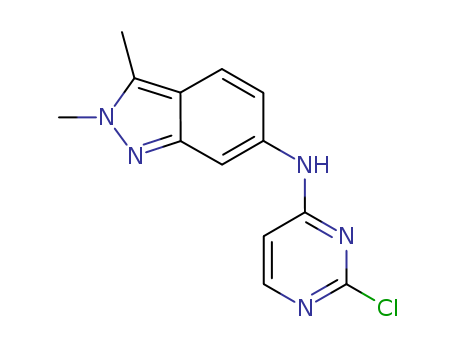
- Product Name:N-(2-Chloropyrimidin-4-YL)-2,3-dimethyl-2H-indazol-6-amine
- Molecular Formula:C13H12 Cl N5
- Purity:99%
- Molecular Weight:273.725
Product Details
Chinese Factory Supply Hot Sale N-(2-Chloropyrimidin-4-YL)-2,3-dimethyl-2H-indazol-6-amine 444731-74-2 In Stock
- Molecular Formula:C13H12 Cl N5
- Molecular Weight:273.725
- Vapor Pressure:0mmHg at 25°C
- Melting Point:>216oC (dec.)
- Boiling Point:549.991oC at 760 mmHg
- PKA:2.56±0.30(Predicted)
- Flash Point:286.424oC
- PSA:55.63000
- Density:1.414g/cm3
- LogP:3.14170
N-(2-chloropyriMidin-4-yl)-2,3-diMethyl-2H- indazol-6-aMine(Cas 444731-74-2) Usage
|
Uses |
N-(2-Chloro-4-pyrimidinyl)-2,3-dimethyl-2H-indazol-6-amine is an impurity of Pazopanib (P210925), an oral angiogenesis inhibitor targeting VEGFR and PDGFR. |
InChI:InChI=1/C13H12ClN5/c1-8-10-4-3-9(7-11(10)18-19(8)2)16-12-5-6-15-13(14)17-12/h3-7H,1-2H3,(H,15,16,17)
444731-74-2 Relevant articles
Discovery of Novel Pazopanib-Based HDAC and VEGFR Dual Inhibitors Targeting Cancer Epigenetics and Angiogenesis Simultaneously
Zang, Jie,Liang, Xuewu,Huang, Yongxue,Jia, Yuping,Li, Xiaoyang,Xu, Wenfang,Chou, C. James,Zhang, Yingjie
, p. 5304 - 5322 (2018)
Herein a novel series of pazopanib hybri...
Preparation, biological & cheminformatics-based assessment of N2,N4-diphenylpyrimidine-2,4-diamine as potential Kinase-targeted antimalarials
Toviwek, Borvornwat,Phuangsawai, Oraphan,Konsue, Adchatawut,Hannongbua, Supa,Riley, Jennifer,Mutter, Nicole,Anderson, Mark,Webster, Lauren,Hallyburton, Irene,Read, Kevin D,Gleeson, M. Paul
, (2021/09/04)
Twenty eight new N2,N4-diphenylpyrimidin...
High Turnover Pd/C Catalyst for Nitro Group Reductions in Water. One-Pot Sequences and Syntheses of Pharmaceutical Intermediates
Gallou, Fabrice,Li, Xiaohan,Lipshutz, Bruce H.,Takale, Balaram S.,Thakore, Ruchita R.
supporting information, p. 8114 - 8118 (2021/10/25)
Commercially available Pd/C can be used ...
Pazopanib structure based HDAC (histone deacetylase) and VEGFR (vascular endothelial growth factor receptor) dual-target inhibitor and preparation method and application thereof
-
Paragraph 0190; 0191; 0192, (2018/03/28)
The invention relates to a pazopanib str...
444731-74-2 Process route
-
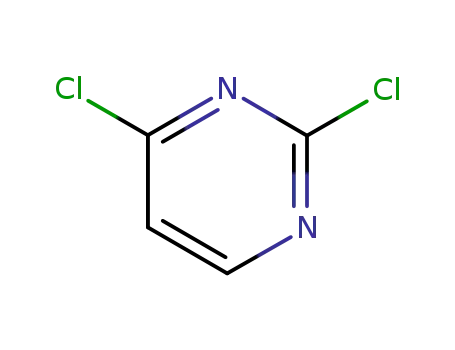
- 3934-20-1
2,6-Dichloropyrimidine

-
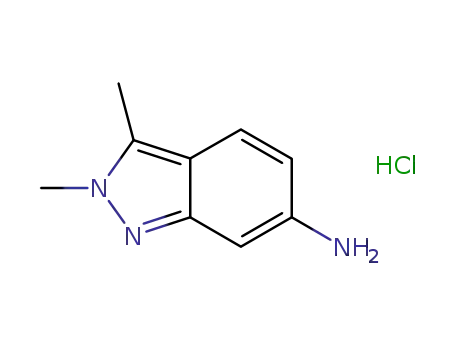
- 635702-60-2
2,3-dimethyl-2H-indazole-6-amine hydrochloride

-
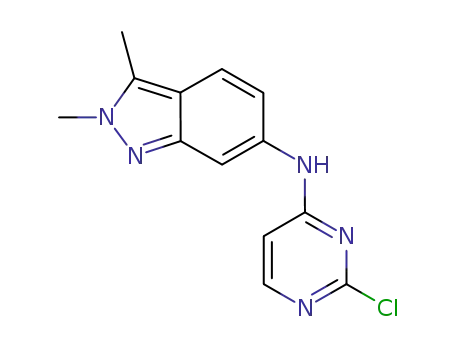
- 444731-74-2
N-(2-chloropyrimidin-4-yl)-2,3-dimethyl-2H-indazol-6-amine
| Conditions | Yield |
|---|---|
|
With sodium hydrogencarbonate; In tetrahydrofuran; ethanol; at 85 ℃; for 4h;
|
89% |
|
With sodium hydrogencarbonate; In tetrahydrofuran; ethanol; at 20 - 85 ℃; for 4h; Inert atmosphere;
|
89% |
-

- 3934-20-1
2,6-Dichloropyrimidine

-
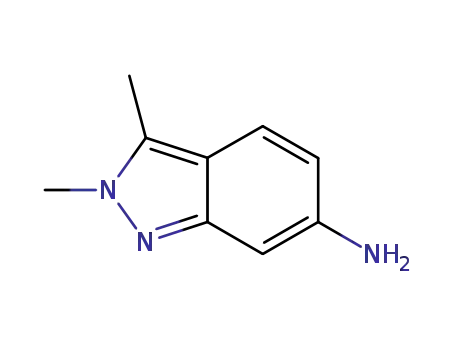
- 444731-72-0
2,3-dimethyl-6-amino-2H-indazole

-

- 444731-74-2
N-(2-chloropyrimidin-4-yl)-2,3-dimethyl-2H-indazol-6-amine
| Conditions | Yield |
|---|---|
|
With sodium hydrogencarbonate; In tetrahydrofuran; ethanol; at 75 ℃;
|
95.2% |
|
2,3-dimethyl-6-amino-2H-indazole; With hydrogenchloride; In 2-methoxy-ethanol; water; at 20 ℃;
2,6-Dichloropyrimidine; With sodium hydrogencarbonate; In tetrahydrofuran; ethanol; at 77 ℃; for 4h;
|
90% |
|
With sodium hydrogencarbonate; In ethanol; at 79 ℃; for 4h;
|
90% |
|
With sodium hydrogencarbonate; In ethanol; at 75 ℃; for 4h;
|
90% |
|
With sodium hydrogencarbonate; In tetrahydrofuran; ethanol; at 85 ℃; for 4h;
|
89% |
|
With sodium hydrogencarbonate; In ethanol; at 85 ℃; for 6h; regioselective reaction;
|
89.5% |
|
With sodium hydrogencarbonate; In methanol; at 25 - 30 ℃; for 24h;
|
86.7% |
|
With sodium hydrogencarbonate; In tetrahydrofuran; ethanol; at 20 - 85 ℃; for 4 - 7h; Product distribution / selectivity;
|
80.1% |
|
With sodium hydrogencarbonate; In isopropyl methanesulfonate; at 85 ℃; for 8h; Product distribution / selectivity; Heating / reflux;
|
80% |
|
With sodium hydrogencarbonate; In tetrahydrofuran; ethanol; at 74 - 76 ℃; for 6 - 7h;
|
80.1% |
|
With sodium hydrogencarbonate; In tetrahydrofuran; ethanol; at 20 - 85 ℃; for 10 - 11h; Product distribution / selectivity;
|
80.1% |
|
With sodium hydrogencarbonate; at 85 ℃; for 8h; Product distribution / selectivity; Heating / reflux;
|
80% |
|
With sodium hydrogencarbonate; In tetrahydrofuran; ethanol; at 20 - 85 ℃; for 4 - 7h; Product distribution / selectivity;
|
80.1% |
|
With sodium hydrogencarbonate; In tetrahydrofuran; ethanol; at 74 - 85 ℃; for 4 - 7h; Product distribution / selectivity;
|
80.1% |
|
With sodium hydrogencarbonate; In tetrahydrofuran; ethanol; at 20 - 85 ℃; for 4 - 7h; Product distribution / selectivity;
|
80.1% |
|
With sodium hydrogencarbonate; In water; at 20 - 85 ℃; for 8h; Product distribution / selectivity; Heating / reflux;
|
80% |
|
With triethylamine; In ethanol; at 70 ℃; for 6h;
|
55.91% |
|
With sodium hydrogencarbonate; In tetrahydrofuran; ethanol; at 0 - 70 ℃; for 7h;
|
1.6% |
|
With sodium hydrogencarbonate; In methanol; ethanol; water; for 8h; Product distribution / selectivity; Reflux; Industry scale; Inert atmosphere;
|
|
|
2,3-dimethyl-6-amino-2H-indazole; With triethylamine; In dimethyl sulfoxide; at 25 - 30 ℃; for 0.166667h;
2,6-Dichloropyrimidine; In dimethyl sulfoxide; at 25 - 30 ℃; for 12h; Solvent; Temperature;
|
70 g |
|
With potassium phosphate; at 45 ℃;
|
54.7 mg |
444731-74-2 Upstream products
-
3934-20-1

2,6-Dichloropyrimidine
-
444731-72-0

2,3-dimethyl-6-amino-2H-indazole
-
635702-60-2

2,3-dimethyl-2H-indazole-6-amine hydrochloride
-
6494-19-5
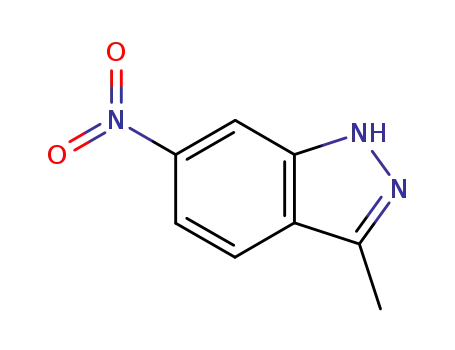
3-methyl-6-nitro-1H-indazole
444731-74-2 Downstream products
-
444731-75-3
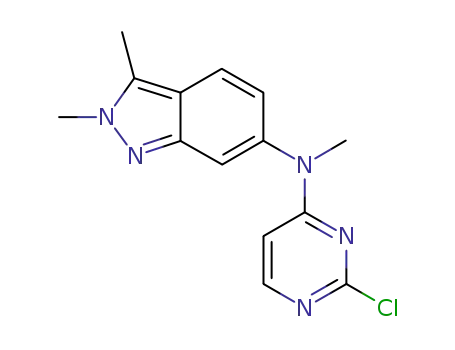
N-(2-chloropyrimidin-4-yl)-N-methyl-2,3-dimethyl-2H-indazole-6-amine
-
1307297-62-6
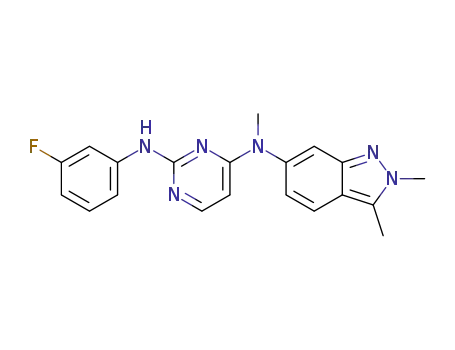
N4-(2,3-dimethyl-2H-indazol-6-yl)-N2-(3-fluorophenyl)-N4-methylpyrimidine-2,4-diamine
-
1307297-61-5
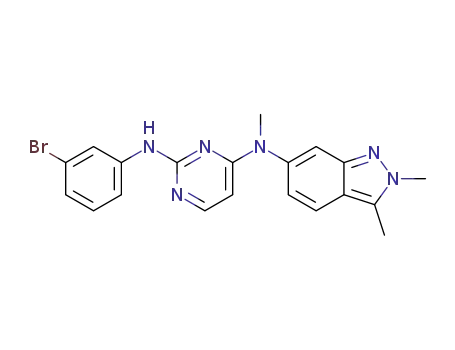
N2-(3-bromophenyl)-N4-(2,3-dimethyl-2H-indazol-6-yl)-N4-methylpyrimidine-2,4-diamine
-
1307297-65-9
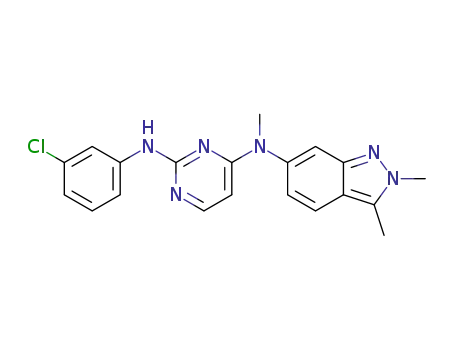
N2-(3-chlorophenyl)-N4-(2,3-dimethyl-2H-indazol-6-yl)-N4-methylpyrimidine-2,4-diamine
Relevant Products
-
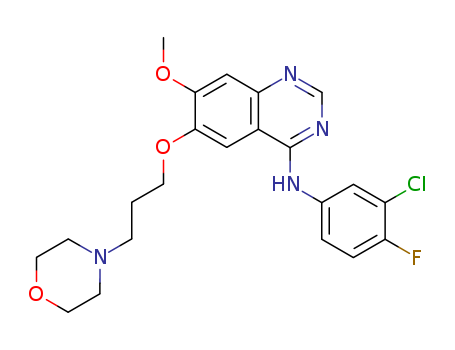
Gefitinib int
CAS:184475-35-2
-
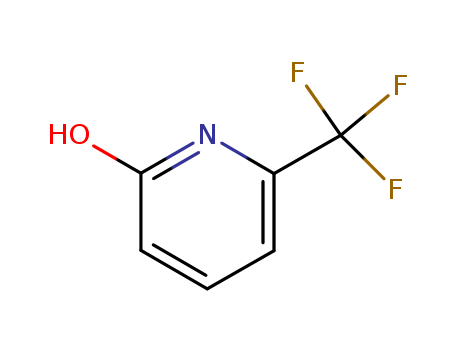
2-Hydroxy-6-(trifluoromethyl)puridine
CAS:34486-06-1
-
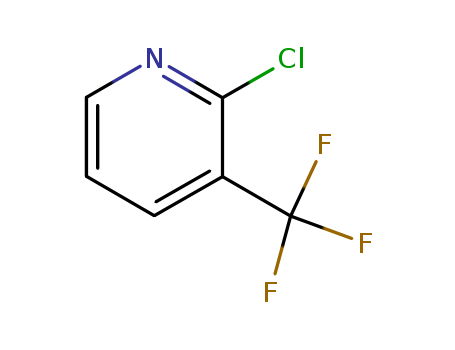
2-Chloro-3-(trifluoromethyl)pyridine
CAS:65753-47-1