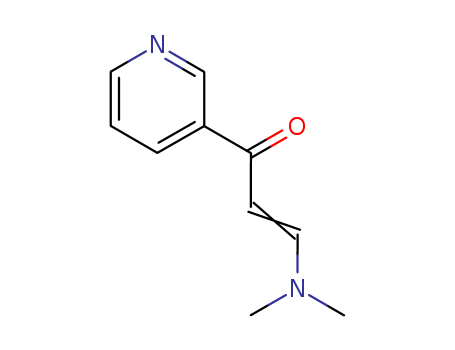
123367-26-0
- Product Name:(E)-3-(Dimethylamino)-1-(3-pyridyl)prop-2-en-1-one
- Molecular Formula:C10H12N2O
- Purity:99%
- Molecular Weight:176.218
Product Details
Chinese Manufacturer Supply 99% Pure (E)-3-(Dimethylamino)-1-(3-pyridyl)prop-2-en-1-one 123367-26-0 with Efficient Transportation
- Molecular Formula:C10H12N2O
- Molecular Weight:176.218
- Melting Point:113℃
- Boiling Point:281℃
- PKA:4.74±0.70(Predicted)
- Flash Point:124℃
- PSA:33.20000
- Density:1.070
- LogP:1.33960
123367-26-0 Relevant articles
Phenylamino-pyrimidine (PAP) - Derivatives: A new class of potent and highly selective PDGF-Receptor autophosphorylation inhibitors
Zimmermann, Juerg,Buchdunger, Elisabeth,Mett, Helmut,Meyer, Thomas,Lydon, Nicholas B.,Traxler, Peter
, p. 1221 - 1226 (1996)
Phenylamino-pyrimidines represent a nove...
Discovery of N-(2-Aminophenyl)-4-[(4-pyridin-3-ylpyrimidin-2-ylamino) methyl]benzamide(MGCD0103), an orally active histone deacetylase inhibitor
Zhou, Nancy,Moradei, Oscar,Raeppel, Stephane,Leit, Silvana,Frechette, Sylvie,Gaudette, Frederic,Paquin, Isabelle,Bernstein, Naomy,Bouchain, Giliane,Vaisburg, Arkadii,Jin, Zhiyun,Gillespie, Jeff,Wang, James,Fournel, Marielle,Yan, Pu T.,Trachy-Bourget, Marie-Claude,Kalita, Ann,Lu, Aihua,Rahil, Jubrail,MacLeod, A. Robert,Li, Zuomei,Besterman, Jeffrey M.,Delorme, Daniel
, p. 4072 - 4075 (2008)
The design, synthesis, and biological ev...
Imatinib intermediate as a two in one dual channel sensor for the recognition of Cu2+ and I- ions in aqueous media and its practical applications
Patil, Samadhan R.,Nandre, Jitendra P.,Jadhav, Devising,Bothra, Shilpa,Sahoo,Devi, Manisha,Pradeep, Chullikkattil P.,Mahulikar, Pramod P.,Patil, Umesh D.
, p. 13299 - 13306 (2014)
An imatinib intermediate, 6-methyl-N-[4-...
Urea derivatives of STI571 as inhibitors of Bcr-Abl and PDGFR kinases
Manley, Paul W.,Breitenstein, Werner,Brüggen, Josef,Cowan-Jacob, Sandra W.,Furet, Pascal,Mestan, Jürgen,Meyer, Thomas
, p. 5793 - 5797 (2004)
Urea-based analogues of STI571 are descr...
New bis-, tris- and tetrakis(pyrazolyl)borate ligands with 3-pyridyl and 4-pyridyl substituents: Synthesis and coordination chemistryt
Adams, Harry,Batten, Stuart R.,Davies, Graham M.,Duriska, Martin B.,Jeffery, John C.,Jensen, Paul,Lu, Jinzhen,Motson, Graham R.,Coles, Simon J.,Hursthouse, Michael B.,Ward, Michael D.
, p. 1910 - 1923 (2005)
The new ligands dihydrobis[3-(4-pyridyl)...
Synthesis and muscarinic activities of 3-(pyrazolyl)-1,2,5,6-tetrahydropyridine derivatives
Plate, Ralf,Plaum, Marc J. M.,De Boer, Thijs,Andrews, John S.,Rae, Duncan R.,Gibson, Sam
, p. 227 - 237 (1996)
A series of 3-(pyrazolyl)-1,2,5,6-tetrah...
Acid-base profiling of imatinib (Gleevec) and its fragments
Szakács, Zoltán,Béni, Szabolcs,Varga, Zoltán,?rfi, László,Kéri, Gy?rgy,Noszál, Béla
, p. 249 - 255 (2005)
The site-specific basicities of imatinib...
Method for preparing N-(5-carboxyl-2-methylphenyl)-4-(3-pyridine)-2-pyrilamine
-
Paragraph 0035; 0036, (2021/05/05)
The invention discloses a method for pre...
Structure-Based Discovery of Pyrimidine Aminobenzene Derivatives as Potent Oral Reversal Agents against P-gp- And BCRP-Mediated Multidrug Resistance
Qiu, Qianqian,Zou, Feng,Li, Huilan,Shi, Wei,Zhou, Daoguang,Zhang, Ping,Li, Teng,Yin, Ziyu,Cai, Zilong,Jiang, Yuxuan,Huang, Wenlong,Qian, Hai
, p. 6179 - 6197 (2021/06/01)
Overexpression of ATP binding cassette (...
Microscale Parallel Synthesis of Acylated Aminotriazoles Enabling the Development of Factor XIIa and Thrombin Inhibitors
Platte, Simon,Korff, Marvin,Imberg, Lukas,Balicioglu, Ilker,Erbacher, Catharina,Will, Jonas M.,Daniliuc, Constantin G.,Karst, Uwe,Kalinin, Dmitrii V.
supporting information, p. 3672 - 3690 (2021/08/07)
Herein we report a microscale parallel s...
Discovery of highly potent tubulin polymerization inhibitors: Design, synthesis, and structure-activity relationships of novel 2,7-diaryl-[1,2,4]triazolo[1,5-a]pyrimidines
Huo, Xian-Sen,Jian, Xie-Er,Ou-Yang, Jie,Chen, Lin,Yang, Fang,Lv, Dong-Xin,You, Wen-Wei,Rao, Jin-Jun,Zhao, Pei-Liang
, (2021/05/10)
By removing 5-methyl and 6-acetyl groups...
123367-26-0 Process route
-
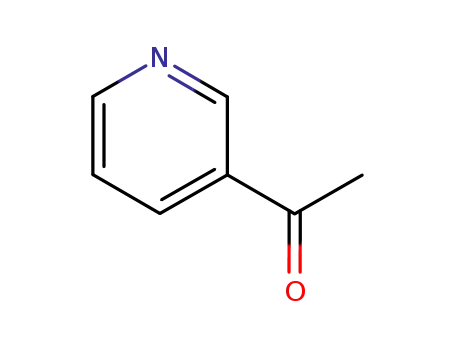
- 350-03-8
methyl-3-pyridylketone

-
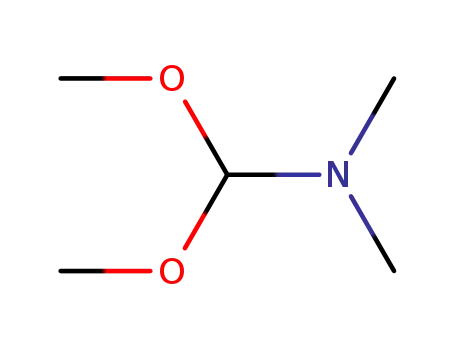
- 4637-24-5
N,N-dimethyl-formamide dimethyl acetal

-
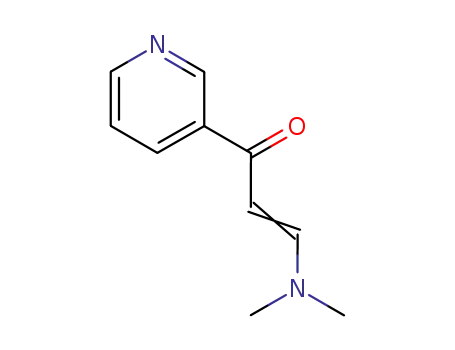
- 55314-16-4,75415-01-9,123367-26-0
3-(N,N-dimethylamino)-1-(pyridin-3-yl)prop-2-en-1-one
| Conditions | Yield |
|---|---|
|
In neat (no solvent); at 65 - 100 ℃;
|
96% |
|
at 85 ℃; for 10h; Large scale;
|
95% |
|
In 5,5-dimethyl-1,3-cyclohexadiene; for 20h; Reflux;
|
94% |
|
In para-xylene; Reflux;
|
94% |
|
at 100 ℃; for 16h; Inert atmosphere;
|
92.6% |
|
With acetic acid; at 90 - 95 ℃; for 2h; Product distribution / selectivity;
|
92% |
|
In 5,5-dimethyl-1,3-cyclohexadiene; at 140 ℃; for 20h; Inert atmosphere; Schlenk technique;
|
92.6% |
|
In 5,5-dimethyl-1,3-cyclohexadiene; at 140 ℃;
|
92% |
|
In 5,5-dimethyl-1,3-cyclohexadiene; at 140 ℃; for 20h;
|
90% |
|
In o-xylene; at 130 ℃; for 20h;
|
90% |
|
In 5,5-dimethyl-1,3-cyclohexadiene; Reflux;
|
87% |
|
Reflux;
|
85% |
|
In neat (no solvent); for 23h; Reflux;
|
85% |
|
In 5,5-dimethyl-1,3-cyclohexadiene; for 21h; Reflux;
|
83.3% |
|
for 4h; Reflux; Neat (no solvent);
|
82% |
|
Reflux;
|
82% |
|
In ethanol; at 20 ℃; for 12h; Reflux;
|
72% |
|
In ethanol; for 12h; Reflux;
|
70% |
|
In ethanol; for 12h; Inert atmosphere; Reflux;
|
70% |
|
In methanol; for 10h; Reflux;
|
60% |
|
In ethanol; at 80 ℃; for 16 - 18h; Heating / reflux;
|
50.8% |
|
In ethanol; Heating;
|
|
|
methyl-3-pyridylketone; N,N-dimethyl-formamide dimethyl acetal; for 23h; Heating / reflux;
at 0 ℃;
|
|
|
In ethanol; Reflux; Inert atmosphere;
|
|
|
In toluene; for 24h; Reflux;
|
|
|
Reflux;
|
|
|
for 6h; Reflux;
|
|
|
In toluene; for 18h; Inert atmosphere; Reflux;
|
|
|
Reflux;
|
|
|
at 100 ℃; for 12h; Inert atmosphere; neat (no solvent);
|
|
|
at 25 - 80 ℃; for 2h; Inert atmosphere;
|
60.2 g |
|
In 5,5-dimethyl-1,3-cyclohexadiene; for 24h; Reflux;
|
|
|
at 170 ℃; for 0.05h; Sealed tube;
|
|
|
In 5,5-dimethyl-1,3-cyclohexadiene; for 48h; Reflux;
|
|
|
In 5,5-dimethyl-1,3-cyclohexadiene; Reflux;
|
|
|
In toluene; at 120 ℃; for 0.25h; Microwave irradiation;
|
|
|
at 110 ℃;
|
|
|
for 16h; Heating / reflux;
|
|
|
for 16h; Heating / reflux;
|
|
|
for 16h; Heating / reflux;
|
|
|
for 24h; Reflux;
|
6.9 g |
|
at 82 - 84 ℃; for 8h;
|
|
|
at 103 ℃;
|
-

- 350-03-8
methyl-3-pyridylketone

-
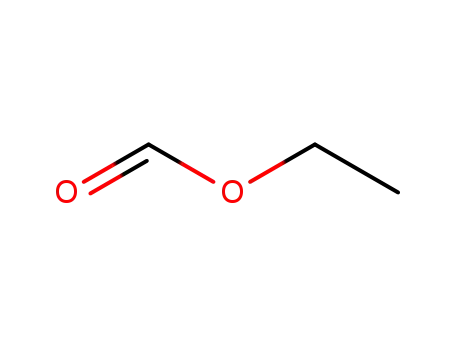
- 109-94-4
formic acid ethyl ester

-

- 55314-16-4,75415-01-9,123367-26-0
3-(N,N-dimethylamino)-1-(pyridin-3-yl)prop-2-en-1-one
| Conditions | Yield |
|---|---|
|
With sodium; acetic acid; dimethyl amine; In methanol; hexane; toluene;
|
123367-26-0 Upstream products
-
350-03-8

methyl-3-pyridylketone
-
4637-24-5

N,N-dimethyl-formamide dimethyl acetal
-
109-94-4

formic acid ethyl ester
-
1188-33-6
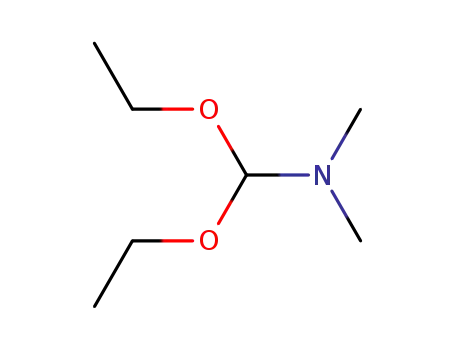
N,N-dimethylformamide diethyl diacetal
123367-26-0 Downstream products
-
152460-09-8
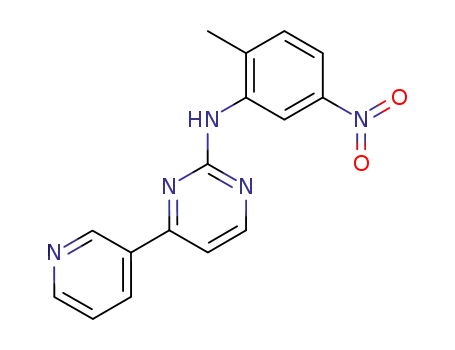
N-(5-nitro-2-methylphenyl)-4-(3-pyridinyl)-2-pyrimidineamine
-
152459-95-5
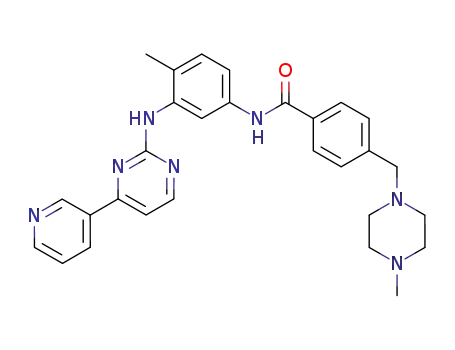
imatinib
-
66521-66-2
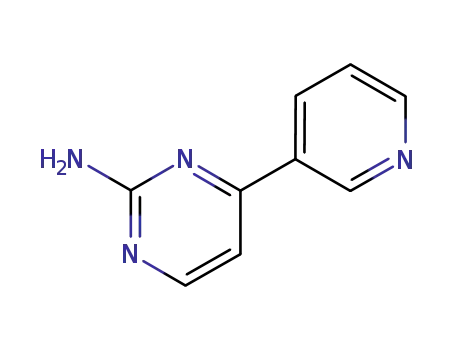
4-pyridin-3-ylpyrimidin-2-ylamine
-
152460-10-1
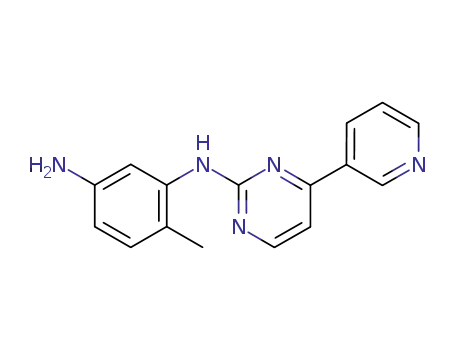
6-methyl-1-N-(4-(pyridin-3-yl)pyrimidin-2-yl)benzene-1,3-diamine
Relevant Products
-
Nilotinib int
CAS:641571-10-0
-
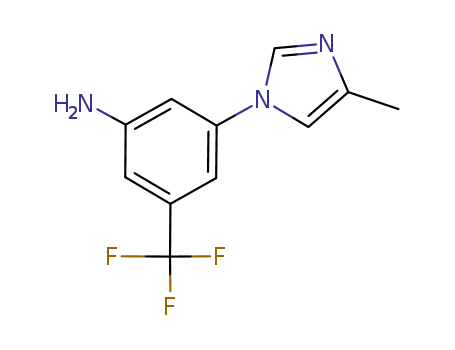
3-(4-Methyl-1H-imidazol-1-yl)-5-(trifluoromethyl)aniline
CAS:641571-11-1
-
1-(3-Pyridyl)-3-(dimethylamino)-2-propen-1-one
CAS:55314-16-4