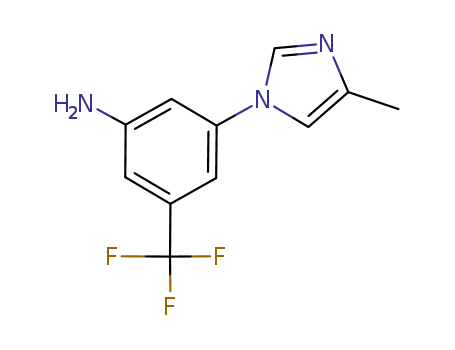
641571-11-1
- Product Name:3-(4-Methyl-1H-imidazol-1-yl)-5-(trifluoromethyl)aniline
- Molecular Formula:C11H10F3N3
- Purity:99%
- Molecular Weight:241.216
Product Details
Reputable Factory Supply Top Purity 3-(4-Methyl-1H-imidazol-1-yl)-5-(trifluoromethyl)aniline 641571-11-1 with Efficient Shipping
- Molecular Formula:C11H10F3N3
- Molecular Weight:241.216
- Vapor Pressure:0mmHg at 25°C
- Melting Point:124-126°C
- Refractive Index:1.553
- Boiling Point:379.805 °C at 760 mmHg
- Flash Point:183.5 °C
- PSA:43.84000
- Density:1.35 g/cm3
- LogP:3.36290
3-(4-Methyl-1H-imidazol-1-yl)-5-(trifluoromethyl)aniline(Cas 641571-11-1) Usage
|
Chemical Properties |
Beige Solid |
InChI:InChI=1/C11H10F3N3/c1-7-5-17(6-16-7)10-3-8(11(12,13)14)2-9(15)4-10/h2-6H,15H2,1H3
641571-11-1 Relevant articles
High Turnover Pd/C Catalyst for Nitro Group Reductions in Water. One-Pot Sequences and Syntheses of Pharmaceutical Intermediates
Gallou, Fabrice,Li, Xiaohan,Lipshutz, Bruce H.,Takale, Balaram S.,Thakore, Ruchita R.
supporting information, p. 8114 - 8118 (2021/10/25)
Commercially available Pd/C can be used ...
SYNTHESIS OF 6-METHYL-N1-(4-(PYRIDIN-3-YL)PYRIMIDIN-2-YL)BENZENE-1,3-DIAMINE
-
Page/Page column 40, (2021/04/23)
Processes and useful intermediates for t...
SUBSTITUTED ARYLUREA COMPOUNDS FOR INDUCING APOPTOSIS AND COMPOSITION FOR ANTICANCER COMPRISING THE SAME
-
Paragraph 0141-0145, (2021/08/17)
The present invention relates to a subst...
Method for synthesizing 3-(4-methyl-1H-imidazole-1-yl)-5-(trifluoromethyl) aniline
-
Page/Page column 6-8, (2020/10/14)
The invention relates to a method for sy...
641571-11-1 Process route
-
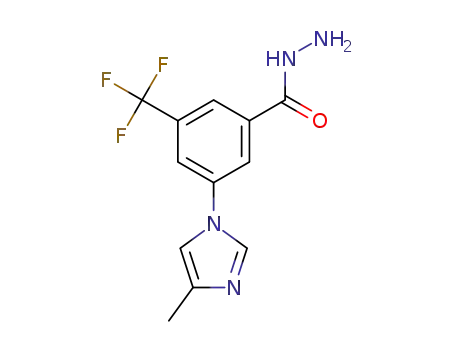
-
3-(4-methyl-1H-imidazol-1-yl)-5-(trifluoromethyl)benzohydrazide

-
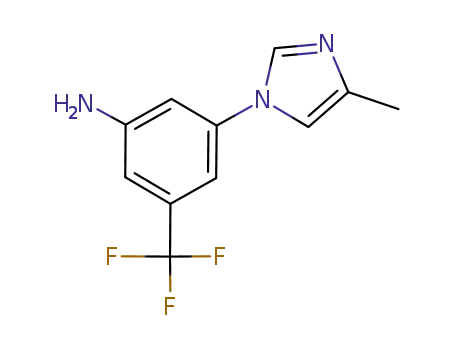
- 641571-11-1
3-(4-methyl-1H-imidazol-1-yl)-5-(trifluoromethyl)aniline
| Conditions | Yield |
|---|---|
|
3-(4-methyl-1H-imidazol-1-yl)-5-(trifluoromethyl)benzohydrazide; With sulfuric acid; sodium nitrite; In methanol; at 15 - 25 ℃; for 0.416667h;
In methanol; at 55 - 75 ℃; for 0.416667h; Concentration; Reagent/catalyst; Temperature;
|
90% |
-
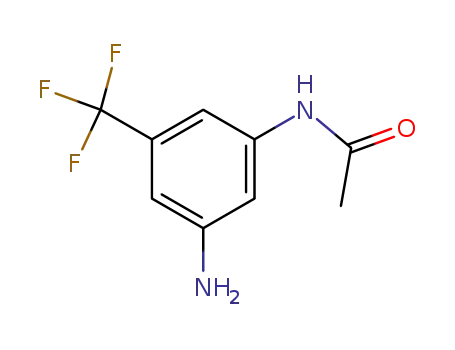
- 455279-96-6
N-(3-amino-5-(trifluoromethyl)phenyl)acetamide

-
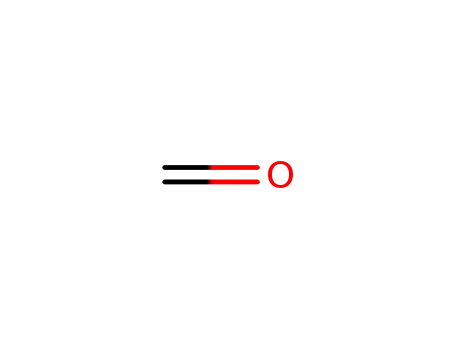
- 50-00-0,30525-89-4,61233-19-0
formaldehyd

-
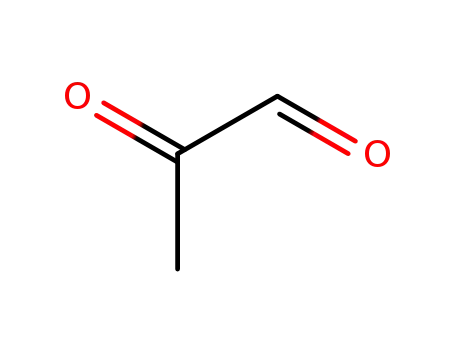
- 78-98-8
2-oxopropanal

-

- 641571-11-1
3-(4-methyl-1H-imidazol-1-yl)-5-(trifluoromethyl)aniline
| Conditions | Yield |
|---|---|
|
N-(3-amino-5-(trifluoromethyl)phenyl)acetamide; With ammonium chloride; In ethanol; acetone; at 0 - 20 ℃;
formaldehyd; 2-oxopropanal; In ethanol; water; acetone; at 65 - 70 ℃;
|
85.4% |
641571-11-1 Upstream products
-
822-36-6
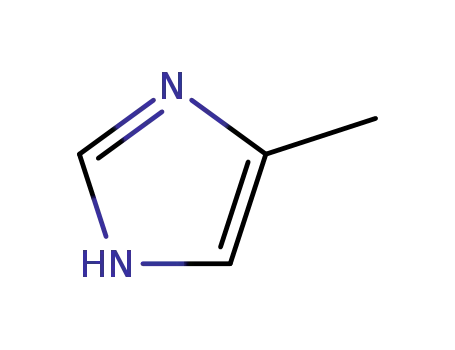
4-methyl-1H-imidazole
-
54962-75-3
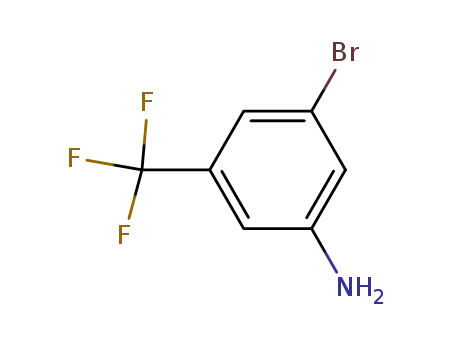
[3-bromo-5-(trifluoromethyl)phenyl]amine
-
916975-92-3
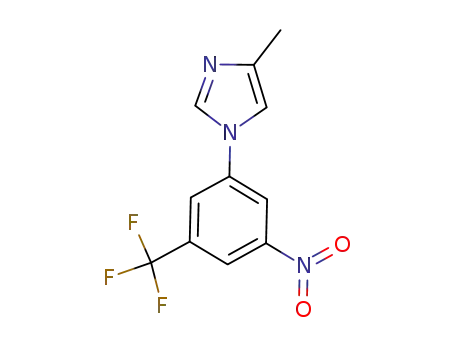
4-methyl-1-(3-nitro-5-(trifluoromethyl)-phenyl)-1H-imidazole
-
917391-26-5
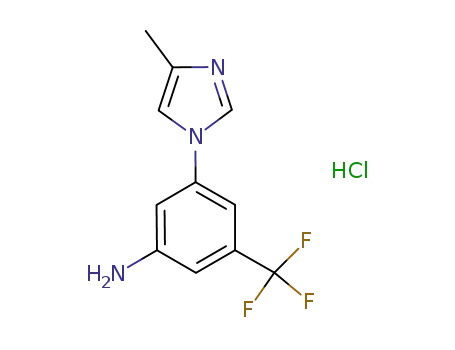
3-(4-methyl-1H-imidazol-1-yl)-5-(trifluoromethyl)aniline hydrochloride
641571-11-1 Downstream products
-
895519-92-3
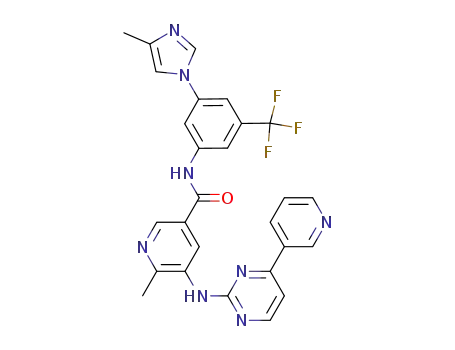
6-methyl-N-{3-(4-methyl-1H-imidazol-1-yl)-5-(trifluoromethyl)phenyl}-5-[4-(pyrid-3-yl)pyrimid-2-yl-amino]nicotinamide
-
926922-18-1
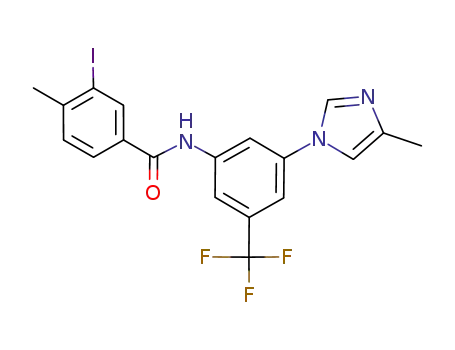
3-iodo-4-methyl-N-[3-(4-methyl-1H-imidazol-1-yl)-5-(trifluoromethyl)phenyl]benzamide
-
641571-10-0
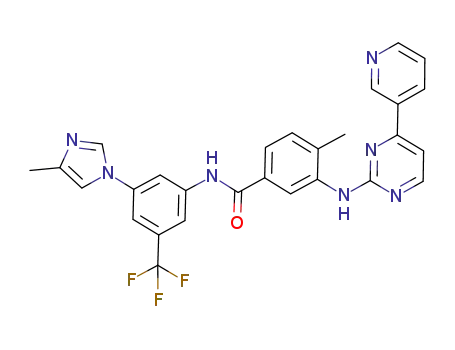
nilotinib
-
1072710-70-3
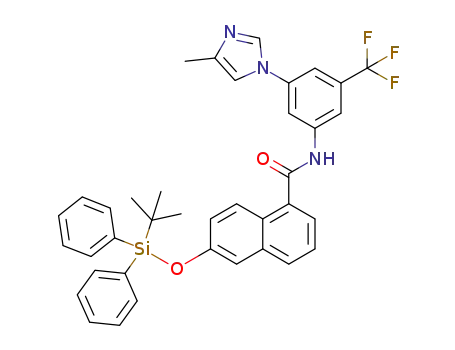
6-[[(1,1-dimethylethyl)diphenylsilyl]oxy]-N-[3-(4-methyl-1H-imidazol-1-yl)-5-(trifluoromethyl)phenyl]-1-naphthalenecarboxamide
Relevant Products
-
Nilotinib int
CAS:641571-10-0
-
4-Methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]benzoic acid
CAS:641569-94-0
-
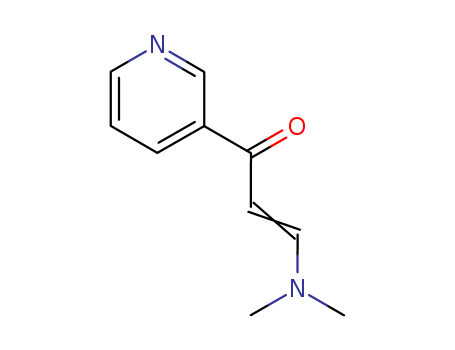
(E)-3-(Dimethylamino)-1-(3-pyridyl)prop-2-en-1-one
CAS:123367-26-0