226256-56-0
- Product Name:CINACALCET int
- Molecular Formula:C22H22F3N
- Purity:99%
- Molecular Weight:357.419
Product Details
Quality Factory Supply Buy High Quality CINACALCET int 226256-56-0 with Safe Transportation
- Molecular Formula:C22H22F3N
- Molecular Weight:357.419
- Appearance/Colour:yellow oil
- Vapor Pressure:5.67E-08mmHg at 25°C
- Refractive Index:1.563
- Boiling Point:440.9 °C at 760 mmHg
- PKA:9.19±0.29(Predicted)
- Flash Point:220.5 °C
- PSA:12.03000
- Density:1.154 g/cm3
- LogP:6.53290
CINACALCET(Cas 226256-56-0) Usage
|
Mechanism |
Cinacalcet is a basic single-chain peptide hormone secreted by the parathyroid chief cells , called PTH, it is a blend of 84 amino acids,it has an effect of elevating serum calcium and lowing phosphorus, regulating calcium and phosphorus metabolism balance of vertebrate body . In parathyroid cells,the first main precursor of PTH is synthesized at first, it is called Prepro-parathyroid, containing 115 amino acids, the predecessor of the substance after cell lysis in the thyroid becomes the second precursor substance,it is called parathyroid-hormone containing 90 amino acids , the latter becomes polypeptide containing 84 amino acids by cleaving within the cell, that is PTH. Normal human plasma PTH concentration is about 1 ng/ml. The main physiological function of parathyroid hormone is promoting osteolysis, bone calcium mobilization into the blood, increasing calcium, increasing vigor of serum and bone alkaline phosphatase ;it can inhibit renal tubular reabsorption of phosphate, promote urinary excretion of phosphorus , decrease phosphorus; PTH via activation of vitamin D3, indirectly promotes intestinal absorption of calcium, magnesium and phosphorus. PTH secretion is mainly affected by the regulation of calcium concentration. Blood Ca2 + elevates, PTH secretion reduces; blood Ca2 + decreases, secretion is increased. In addition, phosphorus increasing via reducing calcium stimulates the secretion of PTH, calcitonin massive release can also promote increasing secretion of PTH. Serum parathyroid hormone except for the study of parathyroid disease, has a certain value on the differential diagnosis of hypercalcemia and hypocalcemia The main clinical significance is as follows: 1. Diagnosis of parathyroid disease. When there are hyperparathyroidism or ectopic PTH secretion disorder, PTH increases ; when there are hypoparathyroidism, parathyroid surgery or radiation damage, etc., PTH decreases. 2. Identification of hypercalcemia and hypocalcemia. When abnormal calcium is caused by calcium parathyroid disorders ,serum calcium lifts as serum PTH lifts, while abnormal blood calcium is caused by other causes , PTH does not change. The above information is edited by the lookchem of Tian Ye. |
|
Description |
Cinacalcet is the first type II calcimimet ic agent approved that improves CaSR sensi Tivity to calcium . When calcium is bound to the CaSR, phospholipase C is act ivated, and the secretion of PTH is inhibited. In the presence of cinacalcet , not only is a drop in PTH levels observed but also a decrease in serum calcium and phosphorous levels. |
|
Chemical Properties |
Yellow Oil |
|
Uses |
Cinacalcet is the first calcimimetic drug approved by the United States Food and Drug Administration for the treatment of secondary hyperparathyroidism in patients with chronic kidney disease |
|
Definition |
ChEBI: A secondary amino compound that is (1R)-1-(naphthalen-1-yl)ethanamine in which one of the hydrogens attached to the nitrogen is substituted by a 3-[3-(trifluoromethyl)phenyl]propyl group. |
|
Brand name |
Sensipar (Amgen). |
|
Clinical Use |
Cinacalcet hydrochloride is a second-generat ion calcimimetic approved for the treatment of secondary hyperparathyroidism in patients wi th chronic kidney disease on dialysis and for the treatment of hypercalcemia in patients with parathyroid cancer . It can be used alone, with vitamin D, and/or with a phosphate binder . |
|
Drug interactions |
Potentially hazardous interactions with other drugs Antifungals: metabolism inhibited by ketoconazole. Hormone antagonists: metabolism of tamoxifen to active metabolite inhibited - avoid. Tobacco: metabolism increased by tobacco. |
|
Metabolism |
Cinacalcet is rapidly and extensively metabolised by cytochrome P450 isoenzymes CYP3A4 and CYP1A2, by oxidation followed by conjugation. The major circulating metabolites are inactive, and are renally excreted, with 80% of the dose recovered in the urine, and 15% in the faeces. |
InChI:InChI=1/C22H22F3N/c1-16(20-13-5-10-18-9-2-3-12-21(18)20)26-14-6-8-17-7-4-11-19(15-17)22(23,24)25/h2-5,7,9-13,15-16,26H,6,8,14H2,1H3/t16-/m1/s1
226256-56-0 Relevant articles
Efficient synthesis of cinacalcet hydrochloride
Bijukumar, Gopinathenpillai,Maloyesh, Biswas,Bhaskar, Bhirud Shekhar,Rajendra, Agarwal
, p. 1512 - 1517 (2008)
A new route to synthesize cinacalcet hyd...
Enantioconvergent Cu-Catalyzed Radical C-N Coupling of Racemic Secondary Alkyl Halides to Access α-Chiral Primary Amines
Cheng, Jiang-Tao,Dong, Xiao-Yang,Gu, Qiang-Shuai,Li, Zhong-Liang,Liu, Juan,Liu, Xin-Yuan,Luan, Cheng,Wang, Fu-Li,Wang, Li-Lei,Yang, Ning-Yuan,Zhang, Yu-Feng
supporting information, p. 15413 - 15419 (2021/09/30)
α-Chiral alkyl primary amines are virtua...
Preparation method of cinacalcet hydrochloride
-
, (2021/05/26)
The invention discloses a method for pre...
Synthesis of Enantioenriched Amines by Iron-Catalysed Amination of Alcohols Employing at Least One Achiral Substrate
Bottari, Giovanni,Afanasenko, Anastasiia,Castillo-Garcia, Antonio A.,Feringa, Ben L.,Barta, Katalin
supporting information, p. 5436 - 5442 (2021/06/17)
The synthesis of a broad range of enanti...
BF3·Et2O as a metal-free catalyst for direct reductive amination of aldehydes with amines using formic acid as a reductant
Fan, Qing-Hua,Liu, Xintong,Luo, Zhenli,Pan, Yixiao,Xu, Lijin,Yang, Ji,Yao, Zhen,Zhang, Xin
supporting information, p. 5205 - 5211 (2021/07/29)
A versatile metal- and base-free direct ...
226256-56-0 Process route
-
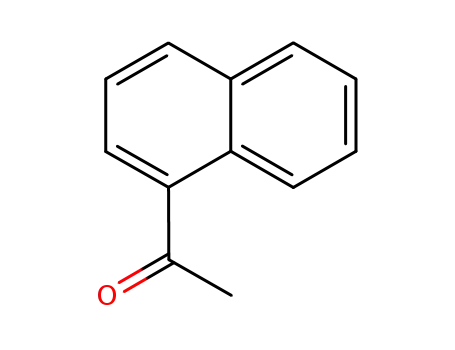
- 941-98-0
1'-naphthacetophenone

-
![3‐[3‐(trifluoromethyl)phenyl]propan‐1‐amine](/upload/2024/4/e96795e3-94e8-4176-b00b-0d16f7fa54e4.png)
- 104774-87-0
3‐[3‐(trifluoromethyl)phenyl]propan‐1‐amine

-
- 226256-56-0,694495-47-1
cinacalcet
| Conditions | Yield |
|---|---|
|
With titanium(IV) isopropylate; hydrogen; 1,8-diazabicyclo<5.4.0>undec-7-ene hidrochloride; In acetic acid methyl ester; at 50 ℃; for 20h; under 38002.6 Torr; Reagent/catalyst; Solvent; stereoselective reaction;
|
94% |
-

- 941-98-0
1'-naphthacetophenone

-
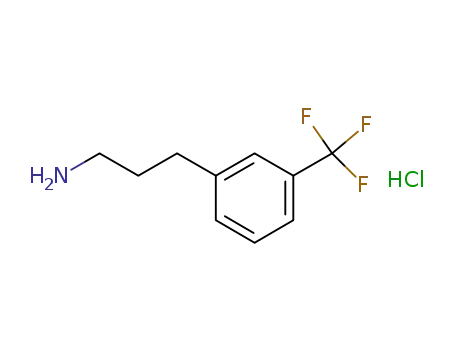
- 104774-93-8
3-(3-trifluoromethyl-phenyl)-propylamine hydrochloride

-

- 226256-56-0,694495-47-1
cinacalcet
| Conditions | Yield |
|---|---|
|
With titanium(IV) isopropylate; hydrogen; In ethyl acetate; at 50 ℃; for 20h; under 38002.6 Torr; stereoselective reaction;
|
91% |
226256-56-0 Upstream products
-
955373-56-5
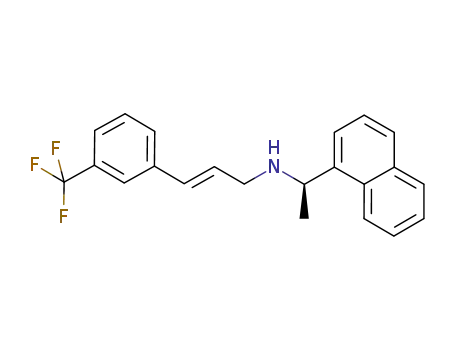
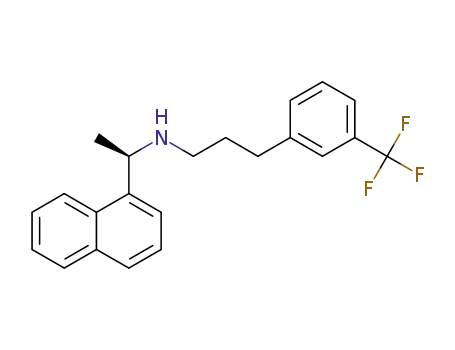
(2E)-N-[(1R)-1-(1-naphthyl)ethyl]-3-[3-(trifluoromethyl)phenyl]prop-2-en-1-amine
-
1005450-55-4
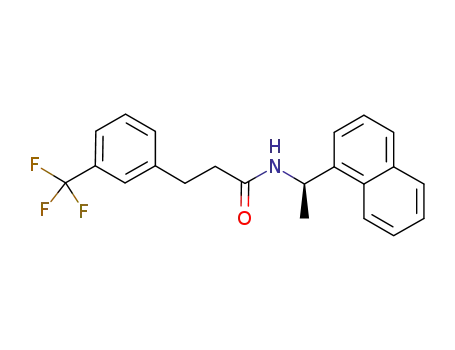
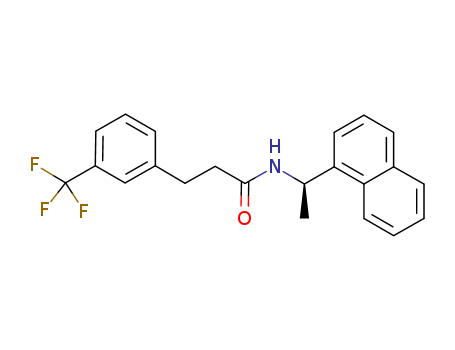
(R)‐N‐(1‐(naphthalen‐1‐yl)ethyl)‐3‐(3‐trifluoromethylphenyl)propanamide
-
364782-34-3
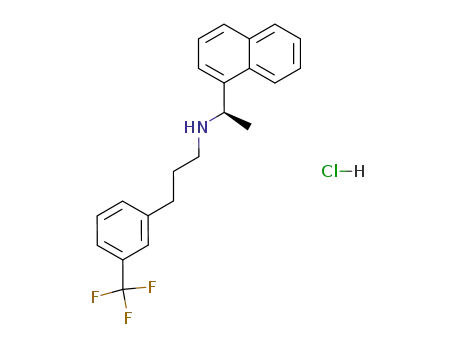
cinacalcet hydrochloride
-
3886-70-2
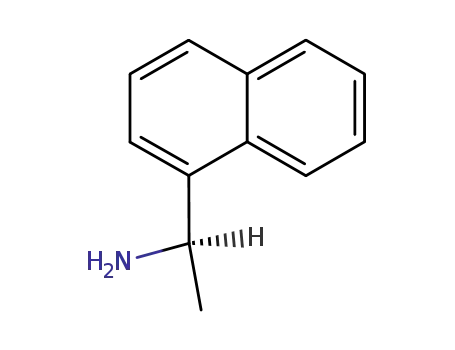
(R)-1-(1-Naphthyl)ethylamine
226256-56-0 Downstream products
-
364782-34-3

cinacalcet hydrochloride
-
1025064-29-2
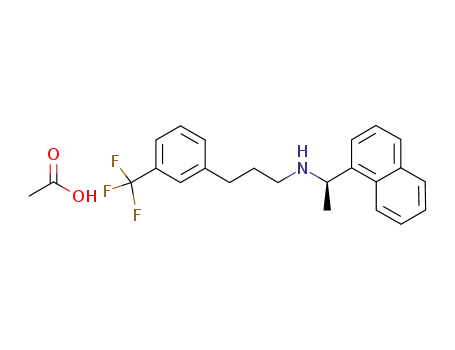
N-[1-(R)-(-)-(1-naphthyl)ethyl]-3-[3-(trifluoromethyl)phenyl]-1-aminopropane acetate
-
1076239-84-3
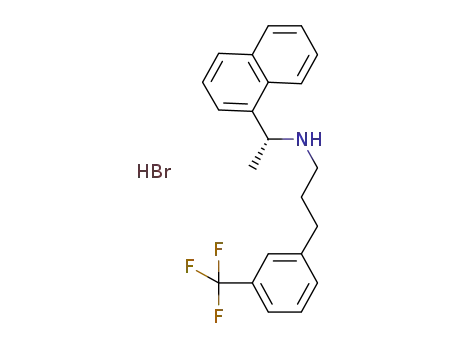
(R)-α-methyl-N-[3-[3-(trifluoromethyl)phenyl]propyl]-1-naphthalenemethaneamine hydrobromide
-
1204313-91-6
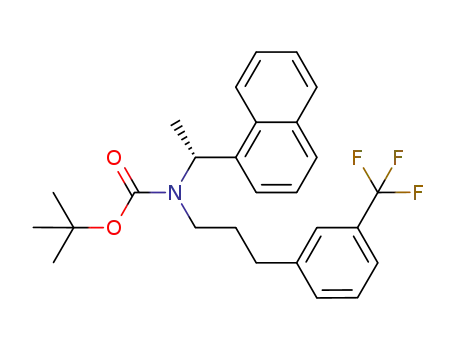
(R)-tert-butyl (1-(naphthalen-1-yl)ethyl)(3-(3-(trifluoromethyl)phenyl)propyl)carbamate
Relevant Products
-
Nilotinib int
CAS:641571-10-0
-
Selumetinib
CAS:606143-52-6