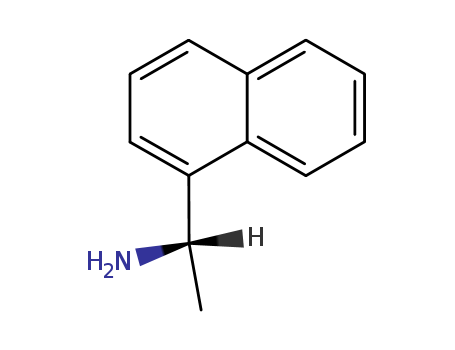
1005450-55-4
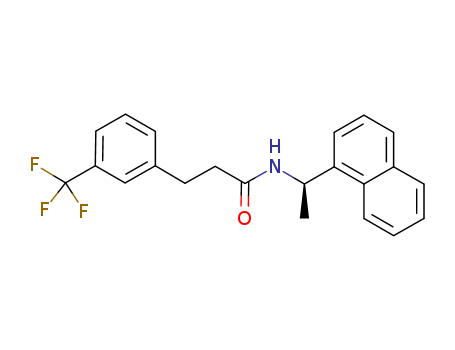
- Product Name:N-((R)-1-(naphthalen-1-yl)ethyl)-3-(3-(trifluoroMethyl)phenyl)propanaMide
- Molecular Formula:C22H20F3NO
- Purity:99%
- Molecular Weight:371.402
Product Details
Reputable Manufacturer Supply High Purity N-((R)-1-(naphthalen-1-yl)ethyl)-3-(3-(trifluoroMethyl)phenyl)propanaMide 1005450-55-4 with Efficient Shipping
- Molecular Formula:C22H20F3NO
- Molecular Weight:371.402
- Melting Point:90-92℃ (cyclohexane )
- Boiling Point:530.5±50.0 °C(Predicted)
- PKA:15.18±0.46(Predicted)
- PSA:29.10000
- Density:1.210±0.06 g/cm3(Predicted)
- LogP:6.05950
1005450-55-4 Relevant articles
Practical synthesis of the calcimimetic agent, cinacalcet
Thiel, Oliver R.,Bernard, Charles,Tormos, Wanda,Brewin, Alan,Hirotani, Shuji,Murakami, Kazuo,Saito, Kenji,Larsen, Robert D.,Martinelli, Michael J.,Reider, Paul J.
, p. 13 - 15 (2008)
A practical synthesis of cinacalcet (Sen...
Enantioselective synthesis of (R)-Cinacalcet via cobalt-catalysed asymmetric Negishi cross-coupling
Sun, Xiao,Wang, Xueyang,Liu, Feipeng,Gao, Zidong,Bian, Qinghua,Wang, Min,Zhong, Jiangchun
, p. 682 - 687 (2019)
A novel enantioselective synthesis of (R...
Method for synthesizing cinacalcet intermediate
-
Paragraph 0007; 0013-0019, (2020/04/02)
The invention particularly discloses a m...
Preparation method of cinacalcet hydrochloride
-
Paragraph 0036; 0038; 0040; 0043-0047, (2020/10/05)
The invention provides a preparation met...
Preparation method of cinacalcet hydrochloride and intermediate thereof
-
, (2020/06/09)
The invention discloses a preparation me...
Synthesis method of cinacalcet hydrochloride intermediate
-
Paragraph 0061-0068, (2020/05/01)
The invention provides a preparation met...
1005450-55-4 Process route
-
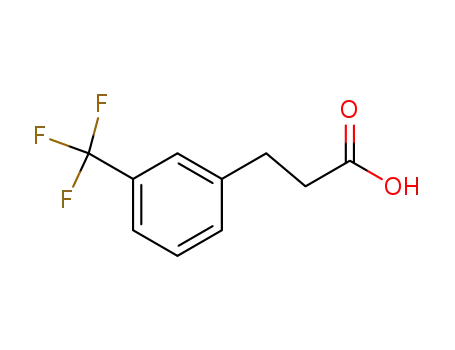
- 585-50-2
3-(3-trifluoromethylphenyl)propanoic acid

-
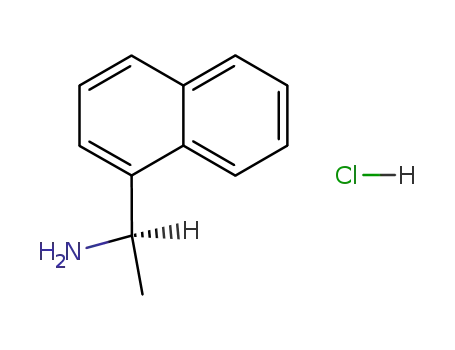
- 82572-04-1
(R)-<1-(1-Naphthyl)ethyl>ammonium chloride

-
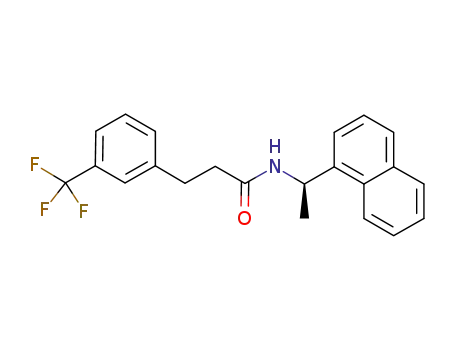
- 1005450-55-4,955371-05-8
(R)‐N‐(1‐(naphthalen‐1‐yl)ethyl)‐3‐(3‐trifluoromethylphenyl)propanamide
| Conditions | Yield |
|---|---|
|
(R)-<1-(1-Naphthyl)ethyl>ammonium chloride; With sodium hydroxide; In toluene;
3-(3-trifluoromethylphenyl)propanoic acid; In toluene; at 140 - 150 ℃; Further stages.;
|
95% |
-
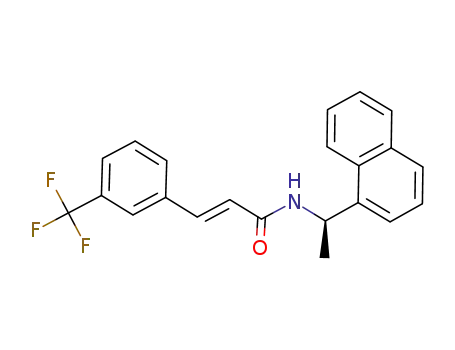
- 1095393-66-0
(R)-N-(1-naphthalen-1-yl-ethyl)-3-(3-trifluoromethyl-phenyl)-(E)-acrylamide

-

- 1005450-55-4,955371-05-8
(R)‐N‐(1‐(naphthalen‐1‐yl)ethyl)‐3‐(3‐trifluoromethylphenyl)propanamide
| Conditions | Yield |
|---|---|
|
With hydrogen; palladium on activated carbon; In methanol; toluene; at 20 ℃; for 1.5h; Product distribution / selectivity;
|
91.2% |
|
With hydrogen; nickel; In methanol; at 45 - 50 ℃; for 3h; under 3677.86 Torr; Product distribution / selectivity;
|
|
|
With hydrogen; palladium on activated charcoal; at 20 ℃; for 3h; under 3677.86 Torr; Product distribution / selectivity;
|
1005450-55-4 Upstream products
-
3886-70-2
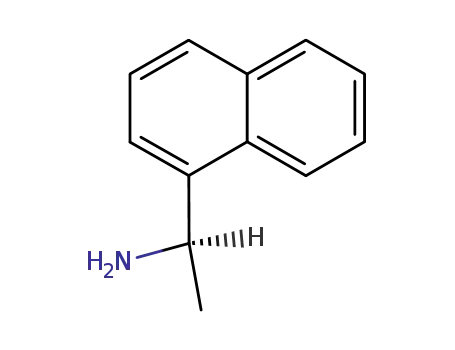
(R)-1-(1-Naphthyl)ethylamine
-
455-03-8
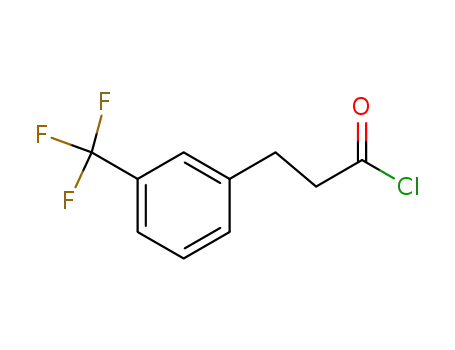
3-(trifluoromethyl)benzenepropanoic acid chloride
-
585-50-2

3-(3-trifluoromethylphenyl)propanoic acid
-
82572-04-1

(R)-<1-(1-Naphthyl)ethyl>ammonium chloride
1005450-55-4 Downstream products
-
226256-56-0
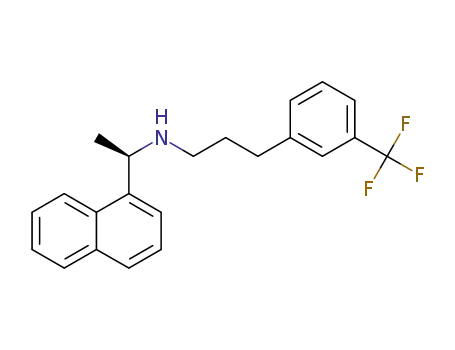
cinacalcet
-
364782-34-3
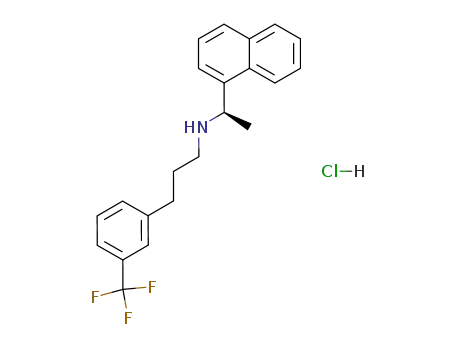
cinacalcet hydrochloride
-
1025064-41-8
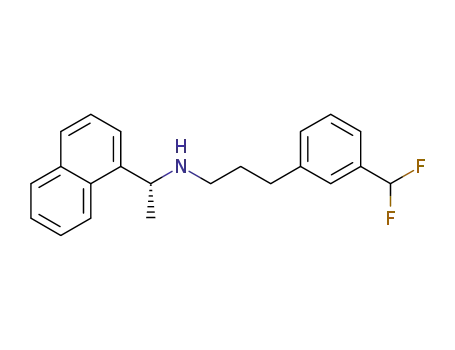
[3-(3-difluoromethyl-phenyl)-propyl]-((R)-1-naphthalen-1-yl-ethyl)-amine
Relevant Products
-
Nilotinib int
CAS:641571-10-0
-
(S)-(-)-1-(1-Naphthyl)ethylamine
CAS:10420-89-0
-
CINACALCET int
CAS:226256-56-0