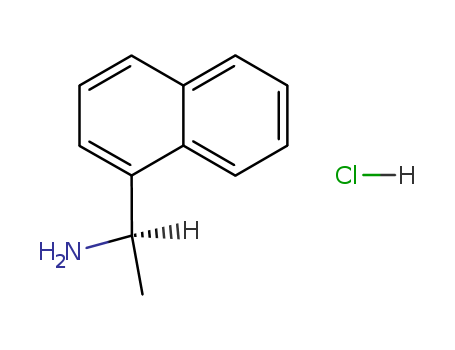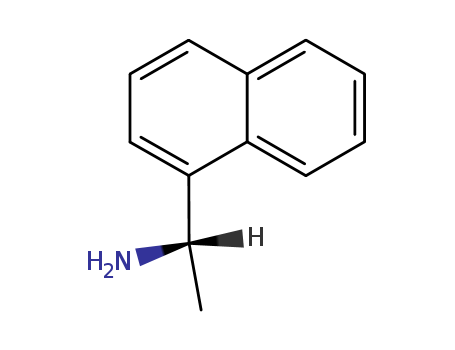
10420-89-0
- Product Name:(S)-(-)-1-(1-Naphthyl)ethylamine
- Molecular Formula:C12H13N
- Purity:99%
- Molecular Weight:171.242
Product Details
Reputable Manufacturer Supply Buy High Quality (S)-(-)-1-(1-Naphthyl)ethylamine 10420-89-0 with Safe Transportation
- Molecular Formula:C12H13N
- Molecular Weight:171.242
- Appearance/Colour:Colorless to light yellow liqui
- Vapor Pressure:0.00214mmHg at 25°C
- Refractive Index:n20/D 1.623(lit.)
- Boiling Point:289.9 °C at 760 mmHg
- PKA:9.26±0.40(Predicted)
- Flash Point:144.3 °C
- PSA:26.02000
- Density:1.067
- LogP:3.55980
(S)-(-)-1-(1-Naphthyl)ethylamine(Cas 10420-89-0) Usage
|
Chemical Properties |
Colorless to light yellow liqui |
|
Uses |
(S)-(-)-1-(1-Naphthyl)ethylamine is used in the asymmetric synthesis of α-cyanocarboxylates. Also used in the synthesis of chiral imadazolin-2-ylidene ligands used in organometallic catalysis. Chiral/ Asymmetric synthesis. |
|
Purification Methods |
Purify the amine by distillation in a good vacuum. [Mori et al. Tetrahedron 37 1343 1981, cf Wilson in Topics Stereochem (Allinger and Eliel eds) v o l 6 135 1971, Fredga et al. Acta Chem Scand 11 1609 1957.] The hydrochlorides crystallise from H2O [] D 18 ±3.9o (c 3, H2O), and the sulfates recrystallise from H2O as tetrahydrates m 230-232o. The RS-amine has b 153o/11mm, 156o/15mm, 183.5o/41mm [Blicke & Maxwell J Am Chem Soc 6 1 1780 1939]. [Beilstein 12 III 3111.] |
InChI:InChI=1/C12H13N/c1-9(13)11-8-4-6-10-5-2-3-7-12(10)11/h2-9H,13H2,1H3/p+1/t9-/m0/s1
10420-89-0 Relevant articles
Asymmetric Supported Reactions: Synthesis Of Chiral Amines
Calmes, Monique,Daunis, Jacques,Hanouneh, Ahmad,Jacquier, Robert
, p. 817 - 820 (1994)
Deracemization of amines, linked via Sch...
Effective Guest Inclusion by a 6-O-Modified β-Cyclodextrin Dimer in Organic Solvents
Asahara, Chizuru,Iwamoto, Takuya,Akashi, Mitsuru,Shigemitsu, Hajime,Kida, Toshiyuki
, p. 868 - 873 (2018)
A 6-O-tert-butyldimethylsilylated β-cycl...
Biocatalytic transamination with near-stoichiometric inexpensive amine donors mediated by bifunctional mono- and di-amine transaminases
Galman, James L.,Slabu, Iustina,Weise, Nicholas J.,Iglesias, Cesar,Parmeggiani, Fabio,Lloyd, Richard C.,Turner, Nicholas J.
, p. 361 - 366 (2017)
The discovery and characterisation of en...
Isopropylidene glycerol hydrogen phthalate: A new resolving agent application to the resolution of 1-arylethylamines
Pallavicini, Marco,Valoti, Ermanno,Villa, Luigi,Piccolo, Oreste
, p. 1117 - 1122 (1996)
Hydrogen phthalates of (R)- and (S)-isop...
Nonenzymatic kinetic resolution of amines in ionic liquids
Sabot, Cyrille,Subhash, Pithani V.,Valleix, Alain,Arseniyadis, Stellios,Mioskowski, Charles
, p. 268 - 272 (2008)
Ionic liquids are remarkably suitable an...
(2-naphthyl)glycolic acid: A tailored resolving agent for p-substituted 1-arylethylamines
Kinbara, Kazushi,Harada, Yoshiko,Saigo, Kazuhiko
, p. 2219 - 2222 (1998)
A tailored resolving agent for p-substit...
n-Butylamine as an alternative amine donor for the stereoselective biocatalytic transamination of ketones
Slabu, Iustina,Galman, James L.,Iglesias, Cesar,Weise, Nicholas J.,Lloyd, Richard C.,Turner, Nicholas J.
, p. 96 - 101 (2018)
Formal reductive amination has been a ma...
Chemoenzymatic Approaches to the Synthesis of the Calcimimetic Agent Cinacalcet Employing Transaminases and Ketoreductases
Marx, Lisa,Ríos-Lombardía, Nicolás,Farnberger, Judith F.,Kroutil, Wolfgang,Benítez-Mateos, Ana I.,López-Gallego, Fernando,Morís, Francisco,González-Sabín, Javier,Berglund, Per
, p. 2157 - 2165 (2018)
Several chemoenzymatic routes have been ...
Efficient kinetic resolution of racemic amines using a transaminase in combination with an amino acid oxidase
Truppo, Matthew D.,Turner, Nicholas J.,Rozzell, J. David
, p. 2127 - 2129 (2009)
A range of enantiomerically pure (R)- an...
Optical resolution and absolute configuration of the chiral pentamethylcyclopentadienylrhenium carbonyl complex 5-C5Me5)Re(NO)(PPh3)(CO)>+BF4-
Huang, Yo-Hsin,Niedercorn, Francine,Arif, Atta M.,Gladysz, J.A.
, p. 213 - 225 (1990)
Reaction of racemic ester (η5-C5Me5)Re(N...
6-TIPS-β-Cyclodextrin-Modified Fe3O4 for Facile Enantioseparation of 1-(1-Naphthyl)ethylamine
Wang, Lu,Liang, Xiang-Yong,Ding, Li-Sheng,Zhang, Sheng,Li, Bang-Jing
, p. 3513 - 3519 (2016)
A new type of chiral magnetic nanopartic...
Highly efficient resolutions with isopropylidene glycerol 3-carboxy-2-naphthoate
Pallavicini, Marco,Bolchi, Cristiano,Fumagalli, Laura,Valoti, Ermanno,Villa, Luigi
, p. 2277 - 2282 (2002)
A number of chiral 1-arylethylamines and...
A METHOD FOR PREPARATION OF DIASTEREOMERIC LACTATE SALTS OF 1-(1-NAPHTHYL)ETHYL AMINE AND PURE ENANTIOMERS OF 1-(1-NAPHTHYL)ETHYL AMINE
-
Paragraph 0043, (2021/09/11)
The invention relates to method for prep...
Method for asymmetrically synthesizing (R)-cinacalcet
-
, (2019/03/28)
The invention discloses a new method for...
Enantioselective synthesis of (R)-Cinacalcet via cobalt-catalysed asymmetric Negishi cross-coupling
Sun, Xiao,Wang, Xueyang,Liu, Feipeng,Gao, Zidong,Bian, Qinghua,Wang, Min,Zhong, Jiangchun
, p. 682 - 687 (2019/08/07)
A novel enantioselective synthesis of (R...
10420-89-0 Process route
-
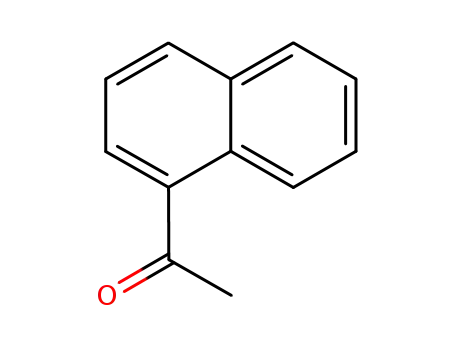
- 941-98-0
1'-naphthacetophenone

-
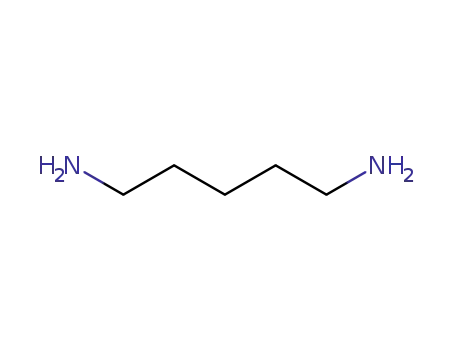
- 462-94-2
1,5-diaminopentane

-
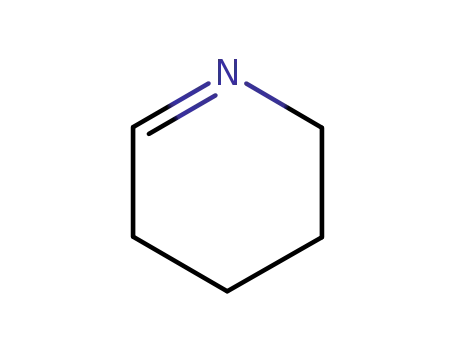
- 505-18-0
2,3,4,5-tetrahydropyridine

-
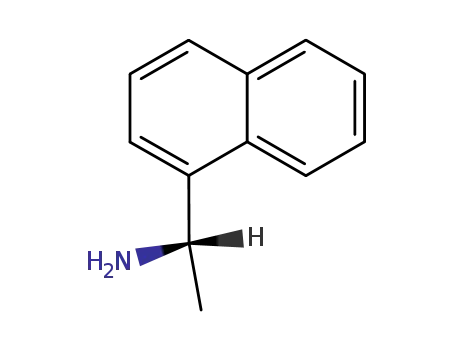
- 10420-89-0
(S)-1-(1-Naphthyl)ethylamine
| Conditions | Yield |
|---|---|
|
With spuC gene from Pseudomonas chlororaphis subsp. aureofaciens; In dimethyl sulfoxide; at 40 ℃; for 24h; pH=9; Reagent/catalyst; enantioselective reaction; Enzymatic reaction;
|
> 99 % ee |
-
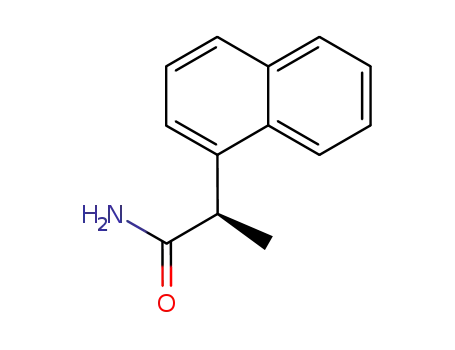
-
(R)‐2‐(1‐naphthalenyl)propanamide

-

- 10420-89-0
(S)-1-(1-Naphthyl)ethylamine

-
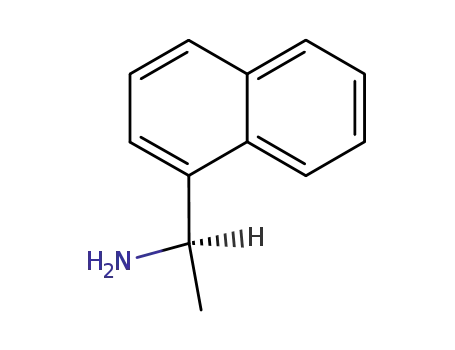
- 3886-70-2
(R)-1-(1-Naphthyl)ethylamine
| Conditions | Yield |
|---|---|
|
With bis-[(trifluoroacetoxy)iodo]benzene; In water; acetonitrile; at 20 ℃; for 24h; Overall yield = 81 %; Overall yield = 313.2 mg;
|
10420-89-0 Upstream products
-
42882-31-5
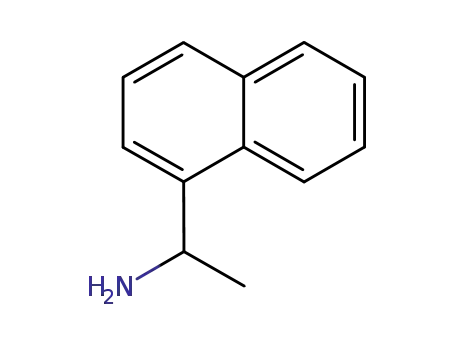
(RS)-1-(1-naphthyl)ethylamine
-
54279-03-7
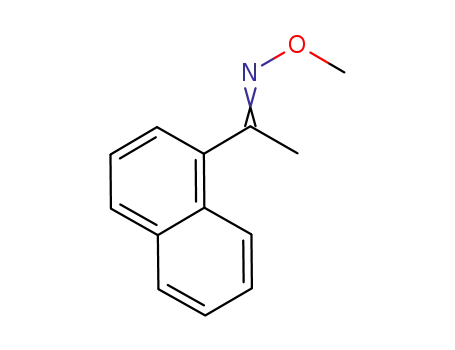
methyl 1-naphthyl ketone O-methyloxime
-
370839-71-7
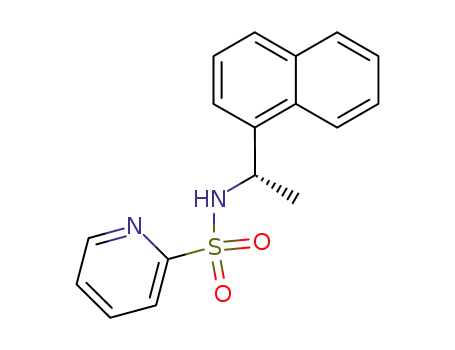
Pyridine-2-sulfonic acid ((S)-1-naphthalen-1-yl-ethyl)-amide
-
141-78-6
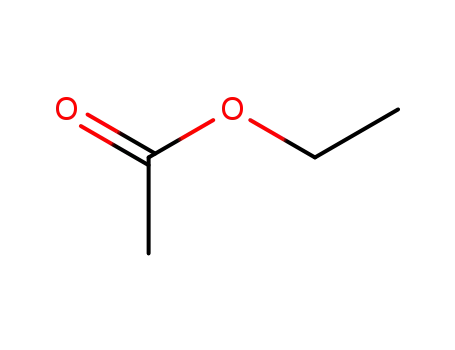
ethyl acetate
10420-89-0 Downstream products
-
64187-99-1
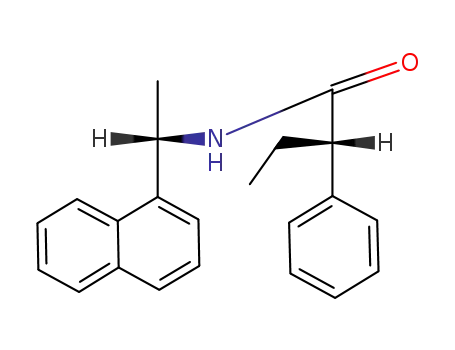
(1S,2'S)-N-<1-(1-naphthyl)ethyl>-2-phenylbutanamide
-
7073-88-3
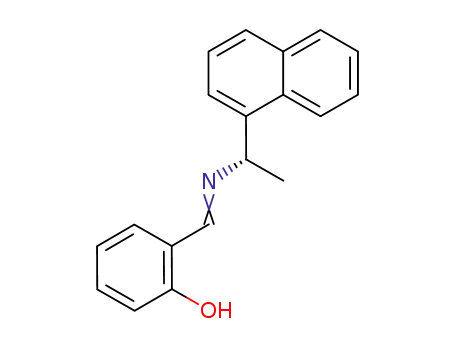
N-salicylidene-(S)-α-maphthylamine
-
95881-32-6
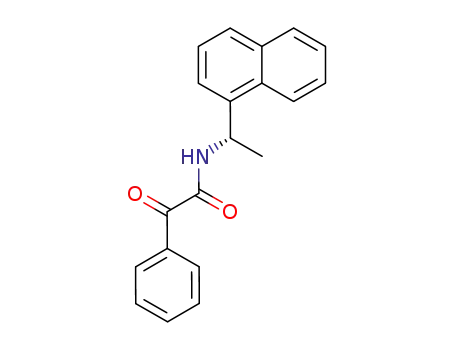
N-<(S)-1-(1-naphthyl)ethyl>benzoylformamide
-
101685-18-1
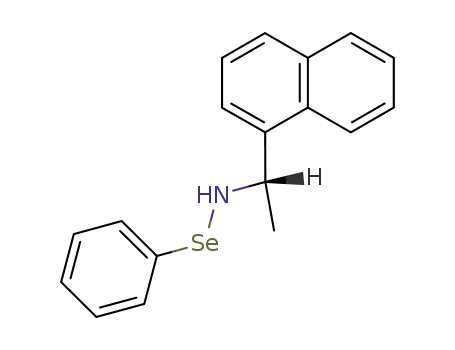
N-<(S)-1-(1-Naphthyl)-ethyl>benzeneselenamide
Relevant Products
-
Nilotinib int
CAS:641571-10-0
-
(R)-(+)-1-(1-NAPHTHYL)ETHYLAMINE HYDROCHLORIDE
CAS:82572-04-1