3886-70-2
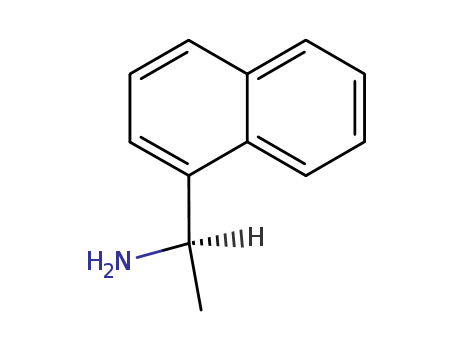
- Product Name:(R)-(+)-1-(1-Naphthyl)ethylamine
- Molecular Formula:C12H13N
- Purity:99%
- Molecular Weight:171.242
Product Details
Quality Factory Supply Hot Sale (R)-(+)-1-(1-Naphthyl)ethylamine 3886-70-2 with Safe Transportation
- Molecular Formula:C12H13N
- Molecular Weight:171.242
- Appearance/Colour:Colorless to light yellow liquid
- Vapor Pressure:0.00214mmHg at 25°C
- Melting Point:135-136 °C
- Refractive Index:n20/D 1.623(lit.)
- Boiling Point:289.9 °C at 760 mmHg
- PKA:9.26±0.40(Predicted)
- Flash Point:144.3 °C
- PSA:26.02000
- Density:1.064 g/cm3
- LogP:3.55980
(R)-(+)-1-(1-Naphthyl)ethylamine(Cas 3886-70-2) Usage
|
Chemical Properties |
Colorless to light yellow liqui |
|
Uses |
(R)-(+)-1-(1-Naphthyl)ethylamine is used in chiral synthesis in organic reactions including the synthesis of β-amino acids and the enantioselective of ketones to nitroolefins. |
|
General Description |
(R)-(+)-1-(1-Naphthyl)ethylamine is a chiral derivatization reagent useful for all gas chromatography (GC) applications in the chiral field. It is specially selected to meet the requirements for derivatization reagents for enantiomeric excess determinations. |
InChI:InChI=1/C12H13N/c1-9(13)11-8-4-6-10-5-2-3-7-12(10)11/h2-9H,13H2,1H3/p+1/t9-/m1/s1
3886-70-2 Relevant articles
A METHOD FOR PREPARATION OF DIASTEREOMERIC LACTATE SALTS OF 1-(1-NAPHTHYL)ETHYL AMINE AND PURE ENANTIOMERS OF 1-(1-NAPHTHYL)ETHYL AMINE
-
Paragraph 0048, (2021/09/11)
The invention relates to method for prep...
Asymmetric reduction method of nitrogen-phosphonyl protected imine
-
Paragraph 0180-0185, (2021/01/15)
The invention discloses an asymmetric re...
Enzymatic Primary Amination of Benzylic and Allylic C(sp3)-H Bonds
Jia, Zhi-Jun,Gao, Shilong,Arnold, Frances H.
supporting information, p. 10279 - 10283 (2020/07/27)
Aliphatic primary amines are prevalent i...
Addition of Highly Polarized Organometallic Compounds to N-tert-Butanesulfinyl Imines in Deep Eutectic Solvents under Air: Preparation of Chiral Amines of Pharmaceutical Interest
Capriati, Vito,Cicco, Luciana,García-álvarez, Joaquín,González-Sabín, Javier,Perna, Filippo M.,Ríos-Lombardía, Nicolás,Salomone, Antonio,Vitale, Paola
, (2020/07/04)
Highly polarized organometallic compound...
3886-70-2 Process route
-
- 10420-89-0
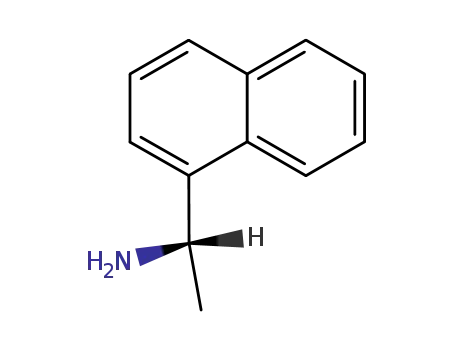
(S)-1-(1-Naphthyl)ethylamine

-
- 100-41-4,27536-89-6
ethylbenzene

-
- 3886-70-2
(R)-1-(1-Naphthyl)ethylamine
| Conditions | Yield |
|---|---|
|
With hydrogen; Pd-BaSO4; In toluene; at 70 ℃; for 24h; under 75.0075 Torr;
|
-
- 42882-31-5
(RS)-1-(1-naphthyl)ethylamine

-

- 3886-70-2
(R)-1-(1-Naphthyl)ethylamine
| Conditions | Yield |
|---|---|
|
With p-chlorophenyl pentanoate; hydrogen; In toluene; at 55 ℃; for 15h; under 75.0075 Torr; Enzymatic reaction;
|
88.3% |
|
With D-(+)-camphoric acid;
|
|
|
Multi-step reaction with 3 steps
1: ethanol / 0.75 h / 60 - 65 °C / Resolution of racemate
2: ammonia / water; toluene
3: potassium hydroxide / dimethyl sulfoxide / 20 h / 150 - 160 °C
With ammonia; potassium hydroxide; In ethanol; water; dimethyl sulfoxide; toluene;
|
|
|
Multi-step reaction with 2 steps
1: water; methanol / 10 h / 60 - 65 °C
2: sodium hydroxide / water; toluene / 0.5 h / 25 - 35 °C / pH 12.5 - 13.5
With sodium hydroxide; In methanol; water; toluene;
|
3886-70-2 Upstream products
-
42882-31-5

(RS)-1-(1-naphthyl)ethylamine
-
371-27-7
2,2,2-trifluoroethylbutyrate
-
121328-40-3
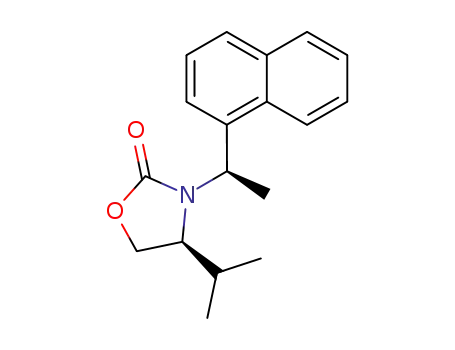
(S)-4-Isopropyl-3-((R)-1-naphthalen-1-yl-ethyl)-oxazolidin-2-one
-
74629-94-0
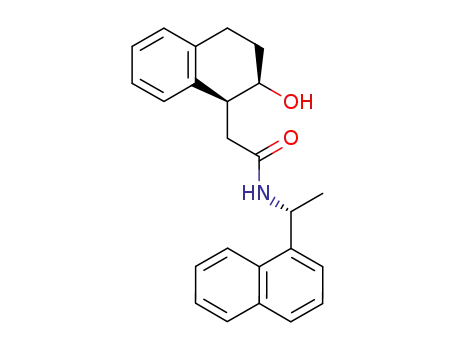
(1S,2R,R)-cis-1-(2-Hydroxy-1,2,3,4-tetrahydro-1-naphthyl)methyl N-<1-(1-naphthyl)ethyl>amide
3886-70-2 Downstream products
-
64187-98-0
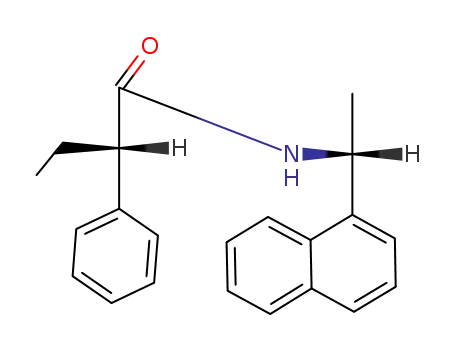
(1R,2'S)-N-<1-(1-naphthyl)ethyl>-2-phenylbutanamide
-
72165-50-5
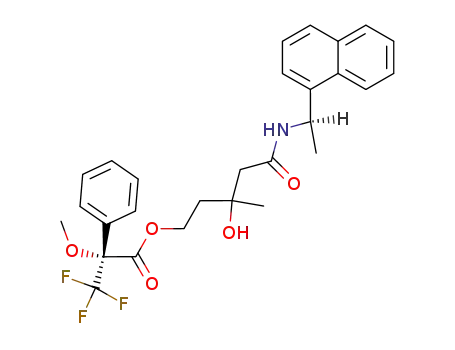
(R)-3,3,3-Trifluoro-2-methoxy-2-phenyl-propionic acid 3-hydroxy-3-methyl-4-((R)-1-naphthalen-1-yl-ethylcarbamoyl)-butyl ester
-
77732-38-8
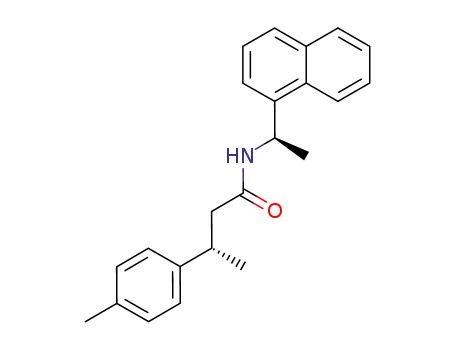
(3S,1'R)-N-(1-naphthylethyl)-3-(4-methylphenyl)butanamide
-
77732-39-9
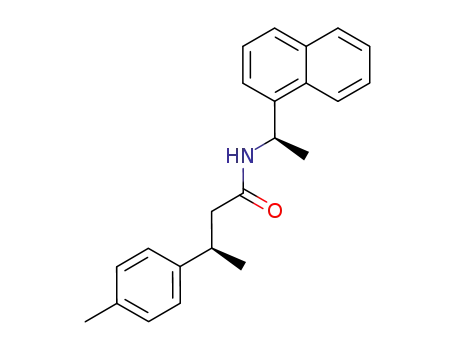
(3R,1'R)-N-(1-naphthylethyl)-3-(4-methylphenyl)butanamide
Relevant Products
-
Nilotinib int
CAS:641571-10-0
-
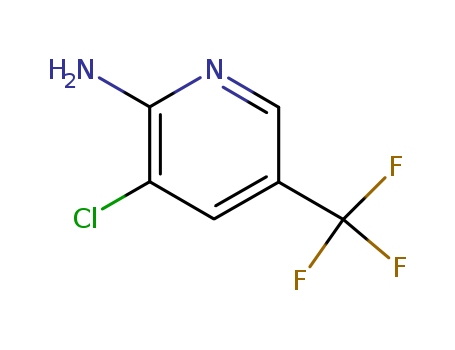
3-Chloro-5-(trifluoromethyl)pyridin-2-amine
CAS:79456-26-1
-
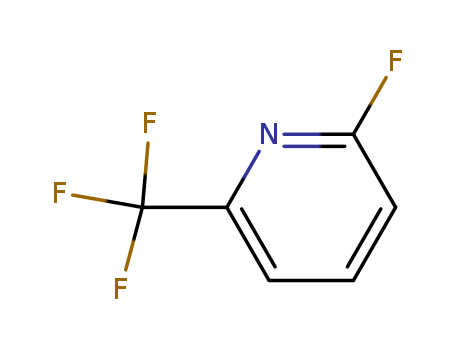
2-fluoro-6-trifluoromethylpyridine
CAS:94239-04-0