779-89-5
- Product Name:3-(Trifluoromethyl)cinnamic acid
- Molecular Formula:C10H7F3O2
- Purity:99%
- Molecular Weight:216.16
Product Details
Trustworthy Factory Supply Buy Reliable Quality 3-(Trifluoromethyl)cinnamic acid 779-89-5 with Reasonable Price
- Molecular Formula:C10H7F3O2
- Molecular Weight:216.16
- Appearance/Colour:white solid
- Vapor Pressure:0.001mmHg at 25°C
- Melting Point:135-137 °C(lit.)
- Boiling Point:289.6 °C at 760 mmHg
- PKA:4.25±0.10(Predicted)
- Flash Point:129 °C
- PSA:37.30000
- Density:1.363 g/cm3
- LogP:2.80320
3-(Trifluoromethyl)cinnamic acid(Cas 779-89-5) Usage
|
Chemical Properties |
White Solid |
|
Uses |
Had sedative hypnotic activity in mice, showing potent inhibition of spontaneous motility. |
InChI:InChI=1/C10H7F3O2/c11-10(12,13)8-3-1-2-7(6-8)4-5-9(14)15/h1-6H,(H,14,15)/p-1/b5-4+
779-89-5 Relevant articles
Dual Nickel/Ruthenium Strategy for Photoinduced Decarboxylative Cross-Coupling of α,β-Unsaturated Carboxylic Acids with Cycloketone Oxime Esters
Gao, Ang,Jiang, Run-Chuang,Liu, Chuang-Chuang,Liu, Qi-Le,Lu, Xiao-Yu,Xia, Ze-Jie
supporting information, p. 8829 - 8842 (2021/06/30)
Herein, a dual nickel/ruthenium strategy...
Metal-Free Hydropyridylation of Thioester-Activated Alkenes via Electroreductive Radical Coupling
Xu, Hehuan,Liu, Jiayu,Nie, Feiyun,Zhao, Xiaowei,Jiang, Zhiyong
, p. 16204 - 16212 (2021/10/25)
An electrochemical hydropyridylation of ...
Meta-substituted piperlongumine derivatives attenuate inflammation in both RAW264.7 macrophages and a mouse model of colitis
Gong, Zhaotang,Liu, Guoyun,Mu, Wenwen,Wang, Ziqing,Yang, Jie
, (2021/11/16)
Piperlongumine (PL) has been showed to h...
Phenanthroline functionalized polyacrylonitrile fiber with Pd(0) nanoparticles as a highly active catalyst for the Heck reaction
Xiao, Jian,Zhang, Haonan,Ejike, Anyaegbu Chima,Wang, Lu,Tao, Minli,Zhang, Wenqin
, (2021/03/03)
A series of polyacrylonitrile fibers (PA...
779-89-5 Process route
-
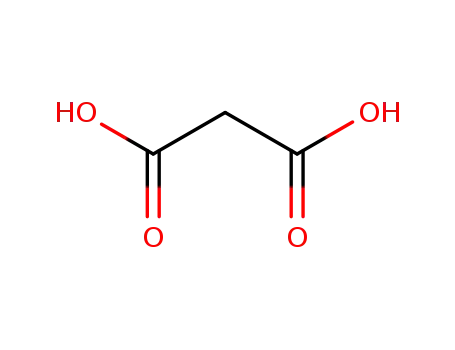
- 141-82-2
malonic acid

-
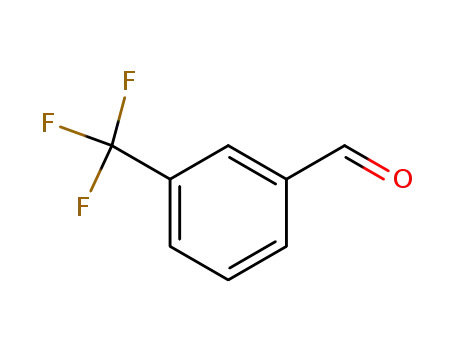
- 454-89-7
3-Trifluoromethylbenzaldehyde

-
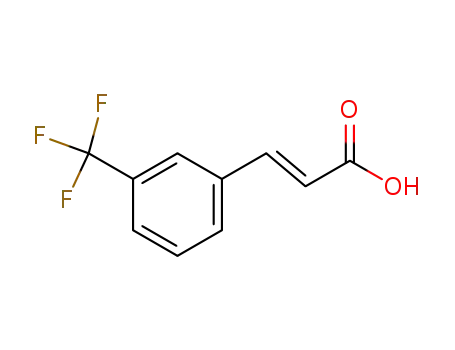
- 779-89-5,67801-07-4
3-(trifluoromethyl)cinnamic acid
| Conditions | Yield |
|---|---|
|
With piperidine; pyridine; at 110 ℃;
|
89% |
|
piperidine; In pyridine; at 110 - 115 ℃;
|
72% |
|
With piperidine; In pyridine; at 80 - 90 ℃;
|
|
|
With piperidine; pyridine; at 100 ℃;
|
|
|
With piperidine; pyridine; for 3h;
|
|
|
With piperidine; pyridine; at 90 ℃; Inert atmosphere;
|
|
|
With piperidine; pyridine; for 3h; Reflux;
|
|
|
With piperidine; pyridine; Reflux;
|
|
|
With piperidine; pyridine; at 115 ℃; for 12h;
|
-
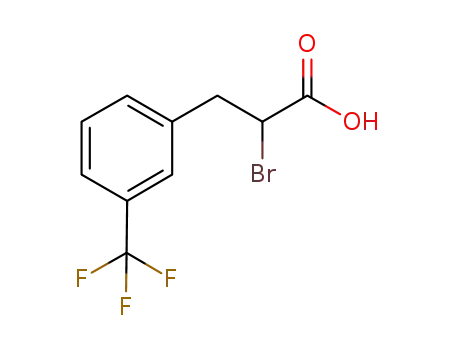
- 1015065-52-7
m-trifluoromethyl-α-bromohydrocinnamic acid

-

- 779-89-5,67801-07-4
3-(trifluoromethyl)cinnamic acid
| Conditions | Yield |
|---|---|
|
m-trifluoromethyl-α-bromohydrocinnamic acid; With sodium hydroxide; water; N-benzyl-N,N,N-triethylammonium chloride; In isopropyl alcohol; at 55 - 60 ℃; for 7h;
With hydrogenchloride; water; at 0 ℃;
|
779-89-5 Upstream products
-
110-89-4
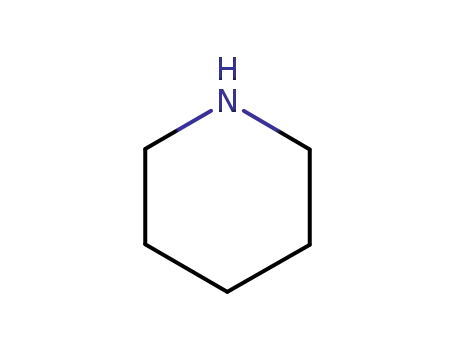
piperidine
-
454-89-7

3-Trifluoromethylbenzaldehyde
-
141-82-2

malonic acid
-
108-24-7
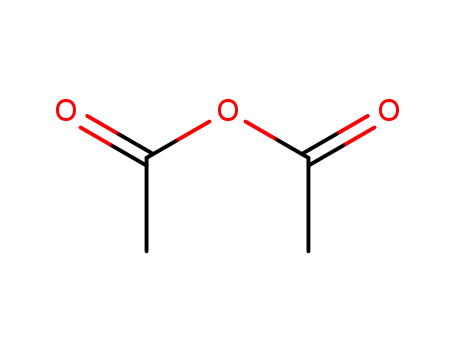
acetic anhydride
779-89-5 Downstream products
-
116577-12-9
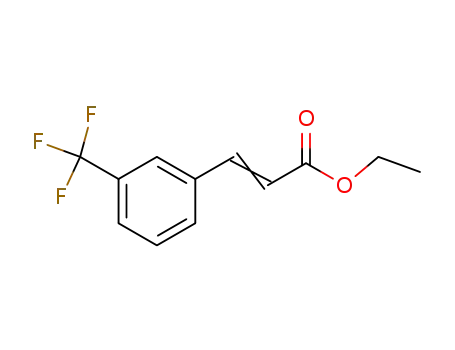
ethyl 3-trifluoromethylcinnamate
-
60689-14-7
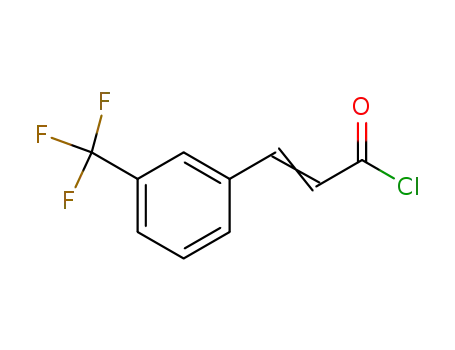
3-(3-(trifluoromethyl)phenyl)acryloyl chloride
-
454-89-7

3-Trifluoromethylbenzaldehyde
-
104774-87-0
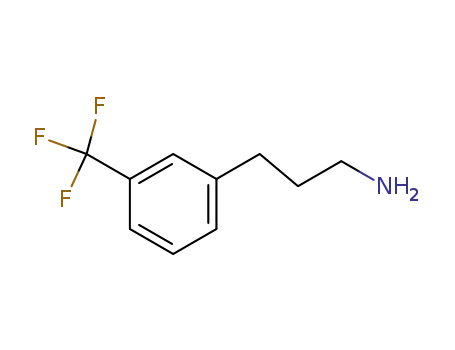
3‐[3‐(trifluoromethyl)phenyl]propan‐1‐amine
Relevant Products
-
Nilotinib int
CAS:641571-10-0
-
3-chloro-2-fluoro-5-(trifluoromethyl)-pyridine
CAS:72537-17-8
-
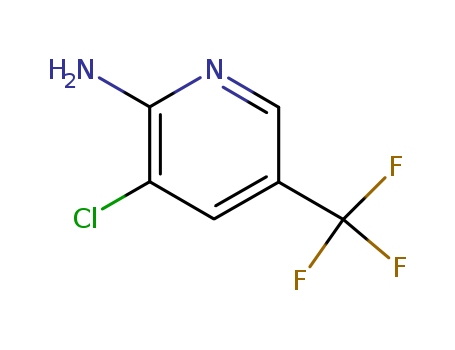
3-Chloro-5-(trifluoromethyl)pyridin-2-amine
CAS:79456-26-1