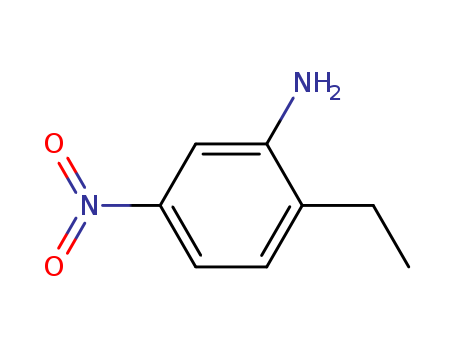
20191-74-6
- Product Name:2-ethyl-5-nitroaniline
- Molecular Formula:C8H10N2O2
- Purity:99%
- Molecular Weight:166.18
Product Details
Factory Supply Buy Quality 2-ethyl-5-nitroaniline 20191-74-6 with Fast Shipping
- Molecular Formula:C8H10N2O2
- Molecular Weight:166.18
- Vapor Pressure:0.000121mmHg at 25°C
- Melting Point:63-64℃ (methanol )
- Refractive Index:1.598
- Boiling Point:335.339 °C at 760 mmHg
- PKA:2.25±0.10(Predicted)
- Flash Point:156.607 °C
- PSA:71.84000
- Density:1.219 g/cm3
- LogP:2.84380
2-Ethyl-5-nitrobenzenamine ,98%(Cas 20191-74-6) Usage
InChI:InChI=1/C8H10N2O2/c1-2-6-3-4-7(10(11)12)5-8(6)9/h3-5H,2,9H2,1H3
20191-74-6 Relevant articles
Preparation method of pazopanib intermediate
-
Paragraph 0185-0187; 0190-0191, (2021/03/24)
The invention provides a preparation met...
Hydrochloric acid [...] of the method for the preparation of intermediates
-
Paragraph 0031; 0032, (2017/01/02)
The invention discloses a preparation me...
2,3-dimethyl-6-urea -2H-indazoles and its preparation method and application
-
Paragraph 0125-0129, (2016/10/09)
The invention discloses a 2, 3-dimethyl-...
Discovery and synthesis of N2,N4-substitued- cycloalkyl[d]pyrimidine-2,4-diamine analogs: The first examples of small-molecular FGFR-1 activator
Li, Bao-Li,Xiao, Fang,Lu, Wen-Chao,Sun, Yu-Yun,Zhu, Jin,Li, Jian
, p. 989 - 994 (2014/08/18)
A series of novel, cycloalkyl-modified p...
20191-74-6 Process route
-
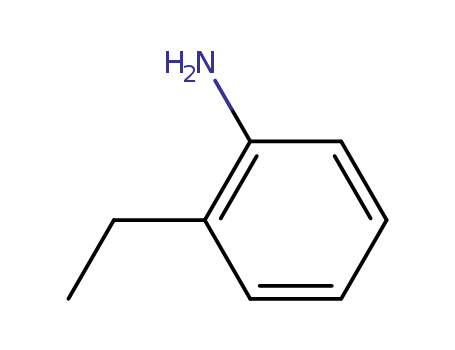
- 578-54-1
ortho-ethylaniline

-
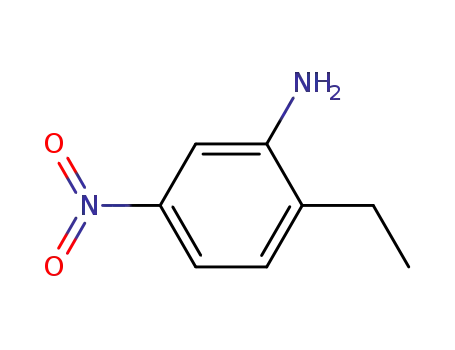
- 20191-74-6
2-ethyl-5-nitroaniline
| Conditions | Yield |
|---|---|
|
With sulfuric acid; nitric acid; at 0 - 20 ℃; for 0.5h;
|
80.1% |
|
With sulfuric acid; nitric acid; at 0 - 5 ℃; for 0.5h;
|
75.3% |
|
With sulfuric acid; nitric acid; at 0 - 5 ℃; for 0.5h;
|
74.1% |
|
With sulfuric acid; nitric acid; for 30h; Ambient temperature;
|
60% |
|
With sulfuric acid; nitric acid;
|
60% |
|
ortho-ethylaniline; With sulfuric acid; nitric acid; at 0 - 20 ℃;
With sodium hydroxide; In water; at 0 ℃;
|
17% |
|
With sulfuric acid; nitric acid;
|
|
|
With sulfuric acid; potassium nitrate;
|
|
|
Multi-step reaction with 2 steps
1: acetic acid
2: concentrated H2SO4; HNO3 / folgenden Hydrolysieren des Reaktionsprodukts
With sulfuric acid; nitric acid; acetic acid;
|
|
|
ortho-ethylaniline; With sulfuric acid; nitric acid; at 20 ℃; for 0.5h;
With sodium hydroxide; In water;
|
|
|
With sulfuric acid; potassium nitrate;
|
|
|
With sulfuric acid; potassium nitrate; at 0 ℃; for 1.5h;
|
-
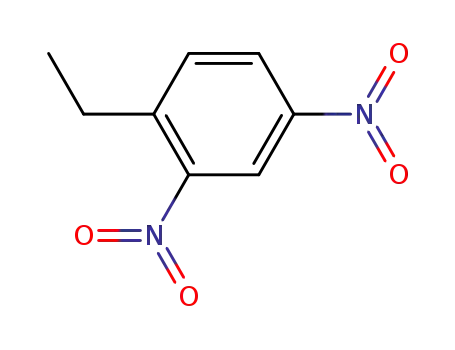
- 1204-29-1
1-ethyl-2,4-dinitrobenzene

-

- 20191-74-6
2-ethyl-5-nitroaniline
| Conditions | Yield |
|---|---|
|
With iron; acetic acid; for 0.166667h; Heating / reflux;
|
51% |
|
With iron; acetic acid; for 0.166667h; Heating / reflux;
|
20191-74-6 Upstream products
-
1204-29-1

1-ethyl-2,4-dinitrobenzene
-
33098-65-6
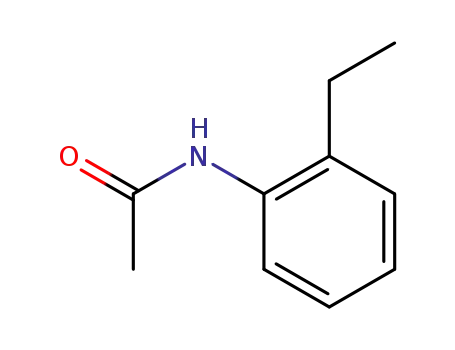
2-acetamido-1-ethylbenzene
-
578-54-1

ortho-ethylaniline
-
612-22-6
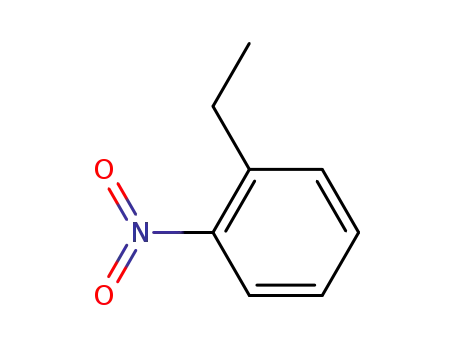
2-nitro(ethylbenzene)
20191-74-6 Downstream products
-
90005-90-6
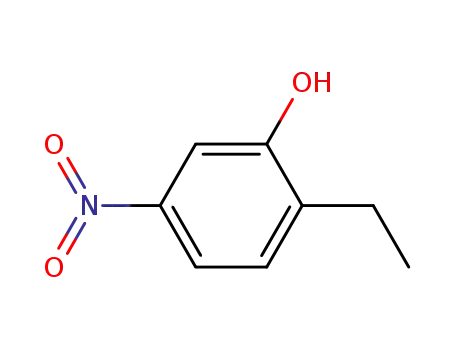
2-ethyl-5-nitro-phenol
-
42782-54-7
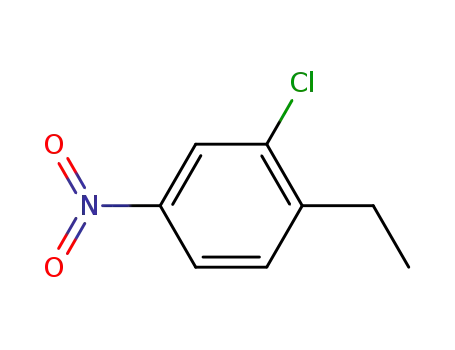
3-Chloro-4-ethylnitrobenzene
-
133053-77-7
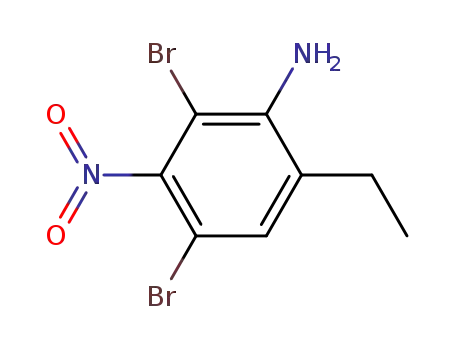
2,4-dibromo-6-ethyl-3-nitroaniline
-
133053-75-5
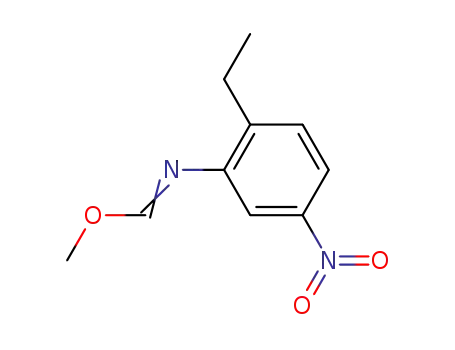
methyl N-(6-ethyl-3-nitrophenyl)-formimidate
Relevant Products
-
Nilotinib int
CAS:641571-10-0
-
Sunitinib Malate int
CAS:341031-54-7