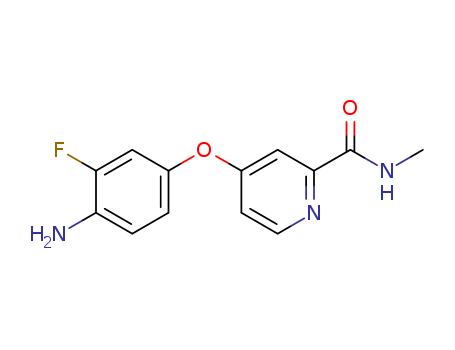
757251-39-1
- Product Name:4-(4-AMINO-3-FLUOROPHENOXY)-N-METHYLPICOLINAMIDE
- Molecular Formula:C13H12 F N3 O2
- Purity:99%
- Molecular Weight:261.256
Product Details
Reputable Manufacturer Supply Top Purity 99% 4-(4-AMINO-3-FLUOROPHENOXY)-N-METHYLPICOLINAMIDE 757251-39-1 with Safe Transportation
- Molecular Formula:C13H12 F N3 O2
- Molecular Weight:261.256
- Vapor Pressure:1.22E-08mmHg at 25°C
- Melting Point:143 °C
- Refractive Index:1.602
- Boiling Point:459.8°C at 760 mmHg
- PKA:13.92±0.46(Predicted)
- Flash Point:231.9°C
- PSA:77.24000
- Density:1.305
- LogP:2.92690
4-(4-AMINO-3-FLUOROPHENOXY)-N-METHYLPICOLINAMIDE(Cas 757251-39-1) Usage
|
Uses |
4-(4-Amino-3-fluorophenoxy)-N-methylpyridine-2-carboxamide is a compound used as a reagent to synthesize Regorafenib (R143000), a multikinase inhibitor that is used to treat patients with advanced colorectal cancer. |
InChI:InChI=1/C13H12FN3O2/c1-16-13(18)12-7-9(4-5-17-12)19-8-2-3-11(15)10(14)6-8/h2-7H,15H2,1H3,(H,16,18)
757251-39-1 Relevant articles
Synthesis method of regorafenib
-
, (2021/04/21)
The invention provides a synthesis metho...
METHODS OF USING REBASTINIB IN THE TREATMENT OF DISORDERS
-
Paragraph 0466; 0469, (2021/09/10)
Described herein are methods of treating...
POLYAROMATIC UREA DERIVATIVES AND THEIR USE IN THE TREATMENT OF MUSCLE DISEASES
-
Page/Page column 123, (2021/01/29)
The current invention provides urea deri...
Regorafenib analogues and their ferrocenic counterparts: Synthesis and biological evaluation
Wilde, Myron,Arzur, Danielle,Baratte, Blandine,Lefebvre, Dorian,Robert, Thomas,Roisnel, Thierry,Le Jossic-Corcos, Catherine,Bach, Stéphane,Corcos, Laurent,Erb, William
, p. 19723 - 19733 (2020/12/04)
Approved by the FDA in 2012, regorafenib...
757251-39-1 Process route
-
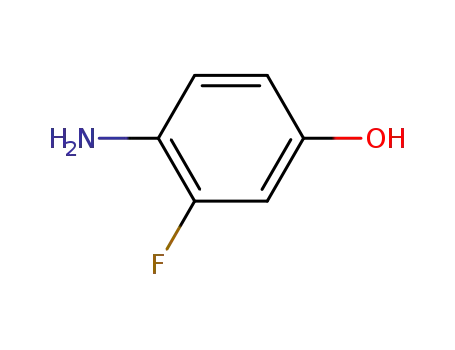
- 399-95-1
4-amino-3-fluorophenol

-
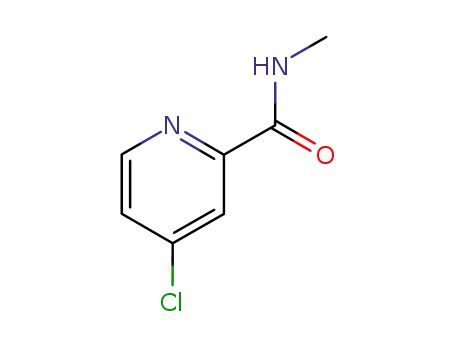
- 220000-87-3
4-chloro-N-methylpicolinamide

-
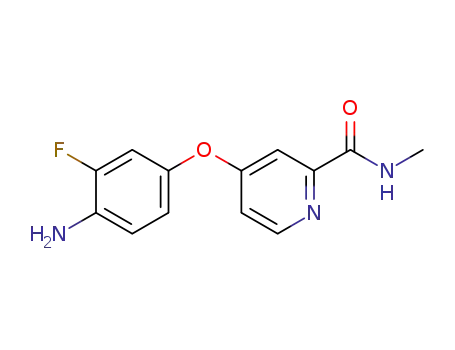
- 757251-39-1
4-(4-amino-3-fluorophenoxy)pyridine-2-carboxylic acid methyl amide
| Conditions | Yield |
|---|---|
|
With potassium carbonate; In 1,2-dichloro-ethane; for 4h; Reflux;
|
96.3% |
|
4-amino-3-fluorophenol; With potassium tert-butylate; In tetrahydrofuran; at 20 - 30 ℃; for 2h;
4-chloro-N-methylpicolinamide; In tetrahydrofuran; Reagent/catalyst; Solvent; Temperature; Reflux;
|
94% |
|
4-amino-3-fluorophenol; With potassium tert-butylate; In dimethyl amine; at 20 ℃; for 2h; Inert atmosphere;
4-chloro-N-methylpicolinamide; With potassium carbonate; In dimethyl amine; at 85 ℃; Time; Inert atmosphere;
|
92% |
|
With sodium hydroxide; In N,N-dimethyl acetamide; at 105 ℃; for 1h; Reagent/catalyst; Solvent; Temperature;
|
88.9% |
|
4-amino-3-fluorophenol; With potassium tert-butylate; In N,N-dimethyl-formamide; at 20 ℃; for 0.5h; Inert atmosphere;
4-chloro-N-methylpicolinamide; In N,N-dimethyl-formamide; at 100 ℃; for 16h; Inert atmosphere;
|
87% |
|
4-amino-3-fluorophenol; With 4-methyl-2-pentanone; for 1h; Reflux;
4-chloro-N-methylpicolinamide; With potassium tert-butylate; In tetrahydrofuran; 1-methyl-pyrrolidin-2-one; at 100 ℃; for 4.16667h; Reagent/catalyst;
|
78% |
|
4-amino-3-fluorophenol; With potassium tert-butylate; In DMA; at 20 ℃; for 0.5h; Sonographic reaction;
4-chloro-N-methylpicolinamide; In DMA; at 100 ℃;
|
77% |
|
4-amino-3-fluorophenol; With potassium tert-butylate; In N,N-dimethyl acetamide; at 20 ℃; for 0.5h; Inert atmosphere;
4-chloro-N-methylpicolinamide; In N,N-dimethyl acetamide; at 100 ℃; Inert atmosphere;
|
77% |
|
4-amino-3-fluorophenol; With potassium tert-butylate; In N,N-dimethyl acetamide; at 15 - 25 ℃; for 0.5h; Inert atmosphere; Sonication;
4-chloro-N-methylpicolinamide; In N,N-dimethyl acetamide; at 100 ℃; Inert atmosphere; Sonication;
|
77% |
|
4-amino-3-fluorophenol; With potassium tert-butylate; In N,N-dimethyl-formamide; for 3.03333h; Inert atmosphere;
4-chloro-N-methylpicolinamide; In N,N-dimethyl-formamide; at 90 ℃; for 10h; Inert atmosphere;
|
54% |
|
With caesium carbonate; In 1-methyl-pyrrolidin-2-one; at 100 ℃; for 12h;
|
29% |
|
4-amino-3-fluorophenol; With potassium tert-butylate; In ISOPROPYLAMIDE; at 0 ℃; for 0.416667h;
4-chloro-N-methylpicolinamide; In ISOPROPYLAMIDE; at 100 ℃; for 16h;
|
|
|
4-amino-3-fluorophenol; With potassium tert-butylate; In ISOPROPYLAMIDE; at 0 ℃; for 0.416667h;
4-chloro-N-methylpicolinamide; In ISOPROPYLAMIDE; at 100 ℃; for 16h;
|
|
|
4-amino-3-fluorophenol; With potassium tert-butylate; In ISOPROPYLAMIDE; at 0 ℃; for 0.416667h;
4-chloro-N-methylpicolinamide; In ISOPROPYLAMIDE; at 100 ℃; for 16h;
|
|
|
4-amino-3-fluorophenol; With potassium tert-butylate; In N,N-dimethyl acetamide; at 0 ℃; for 0.416667h;
4-chloro-N-methylpicolinamide; In N,N-dimethyl acetamide; at 100 ℃; for 16h;
|
|
|
With potassium tert-butylate;
|
|
|
4-amino-3-fluorophenol; With potassium tert-butylate; tetrabutylammomium bromide; potassium carbonate; In acetonitrile; at -15 - 5 ℃; for 0.25h; Inert atmosphere;
4-chloro-N-methylpicolinamide; In acetonitrile; at 5 - 85 ℃; for 6h;
|
25.25 g |
|
With potassium tert-butylate; In tetrahydrofuran; N,N-dimethyl acetamide; at 110 - 115 ℃;
|
9 g |
|
With potassium tert-butylate; In N,N-dimethyl acetamide; at 0 - 90 ℃; for 1.5h;
|
92.9 g |
|
4-amino-3-fluorophenol; 4-chloro-N-methylpicolinamide; In N,N-dimethyl acetamide; at 25 - 30 ℃; Inert atmosphere;
With potassium tert-butylate; In N,N-dimethyl acetamide; at 100 - 110 ℃; Inert atmosphere;
|
|
|
4-amino-3-fluorophenol; With potassium tert-butylate; In N,N-dimethyl-formamide; at 20 ℃; for 1h; Inert atmosphere;
4-chloro-N-methylpicolinamide; In N,N-dimethyl-formamide; at 85 ℃; for 10h; Inert atmosphere;
|
1.3 g |
-
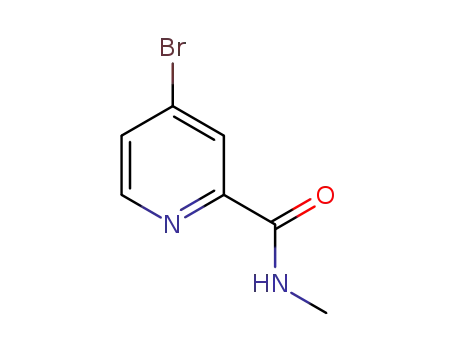
- 1209459-88-0
4-bromo-pyridine-2-carboxylic acid methyl amide

-

- 399-95-1
4-amino-3-fluorophenol

-

- 757251-39-1
4-(4-amino-3-fluorophenoxy)pyridine-2-carboxylic acid methyl amide
| Conditions | Yield |
|---|---|
|
With N-benzyl-N,N,N-triethylammonium chloride; potassium iodide; sodium hydroxide; In water; at 30 - 70 ℃; for 2h; Reagent/catalyst;
|
97.5% |
757251-39-1 Upstream products
-
220000-87-3

4-chloro-N-methylpicolinamide
-
399-95-1

4-amino-3-fluorophenol
-
1338722-52-3
C12H16FNO
-
1338722-53-4
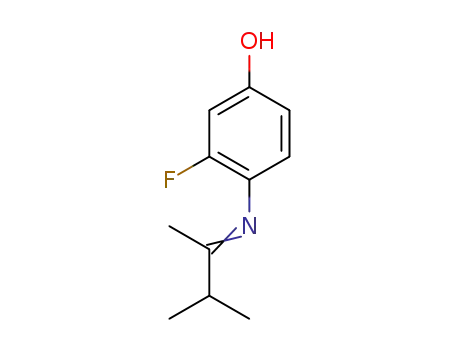
C11H14FNO
757251-39-1 Downstream products
-
755037-03-7
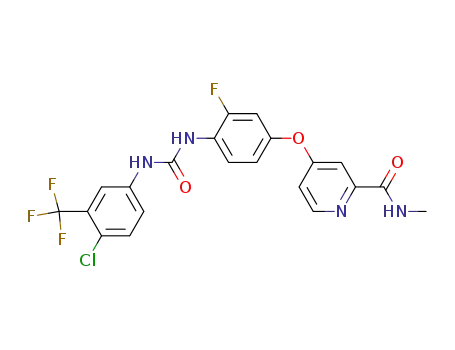
regorafenib
-
1036712-94-3
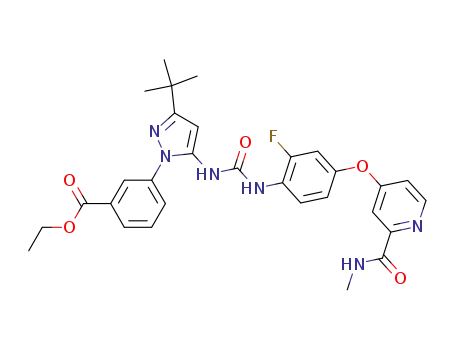
ethyl 3-(3-tert-butyl-5-{[(2-fluoro-4-{[2-(methylcarbamoyl)-pyridin-4-yl]oxy}phenyl)carbamoyl]amino}-1H-pyrazol-1-yl)benzoate
-
1020173-88-9
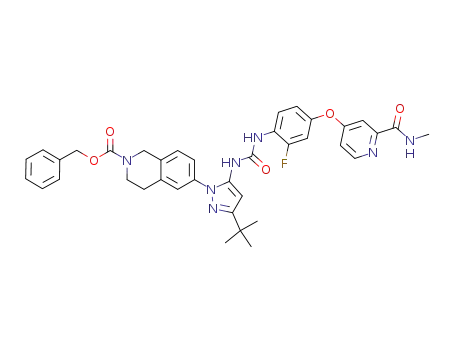
benzyl 6-(3-tert-butyl-5-(3-(2-fluoro-4-(2-(methylcarbamoyl)pyridin-4-yloxy)phenyl)ureido)-1H-pyrazol-1-yl)-3,4-dihydroisoquinoline-2(1H)-carboxylate
-
1020172-07-9
DCC-2036
Relevant Products
-
Nilotinib int
CAS:641571-10-0
-
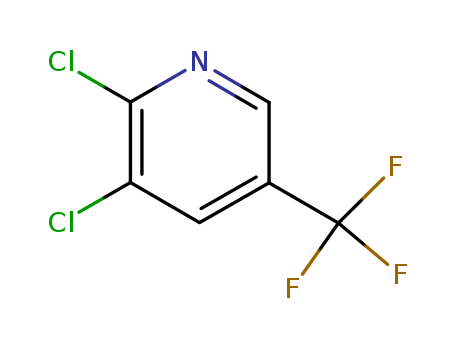
2,3-dichloro-5-(trifluoromethyl)pyridine
CAS:69045-84-7
-
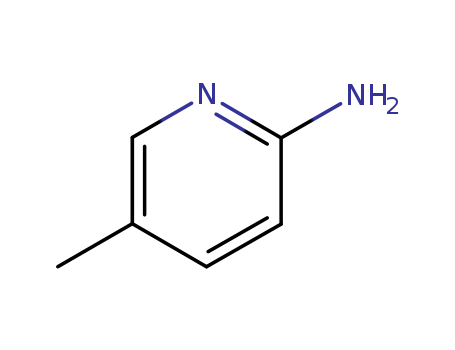
2-Amino-5-methylpyridine
CAS:1603-41-4